Abstract
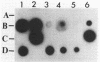
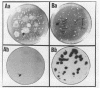
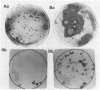
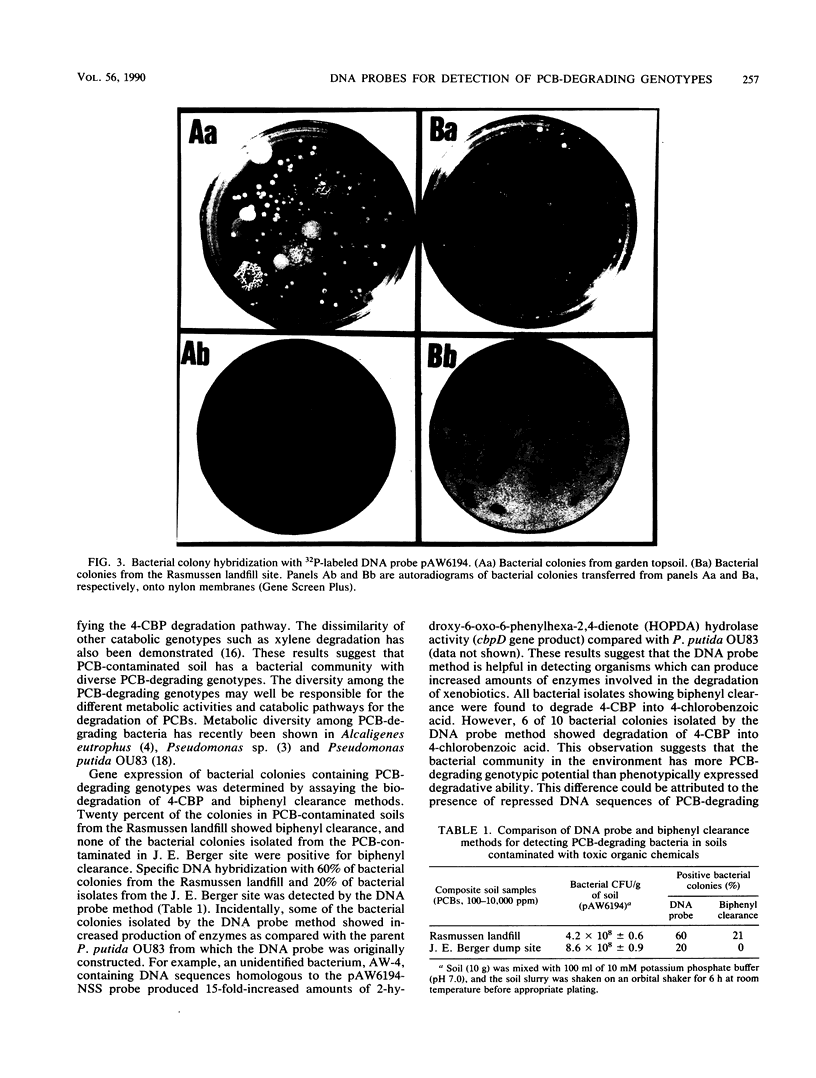
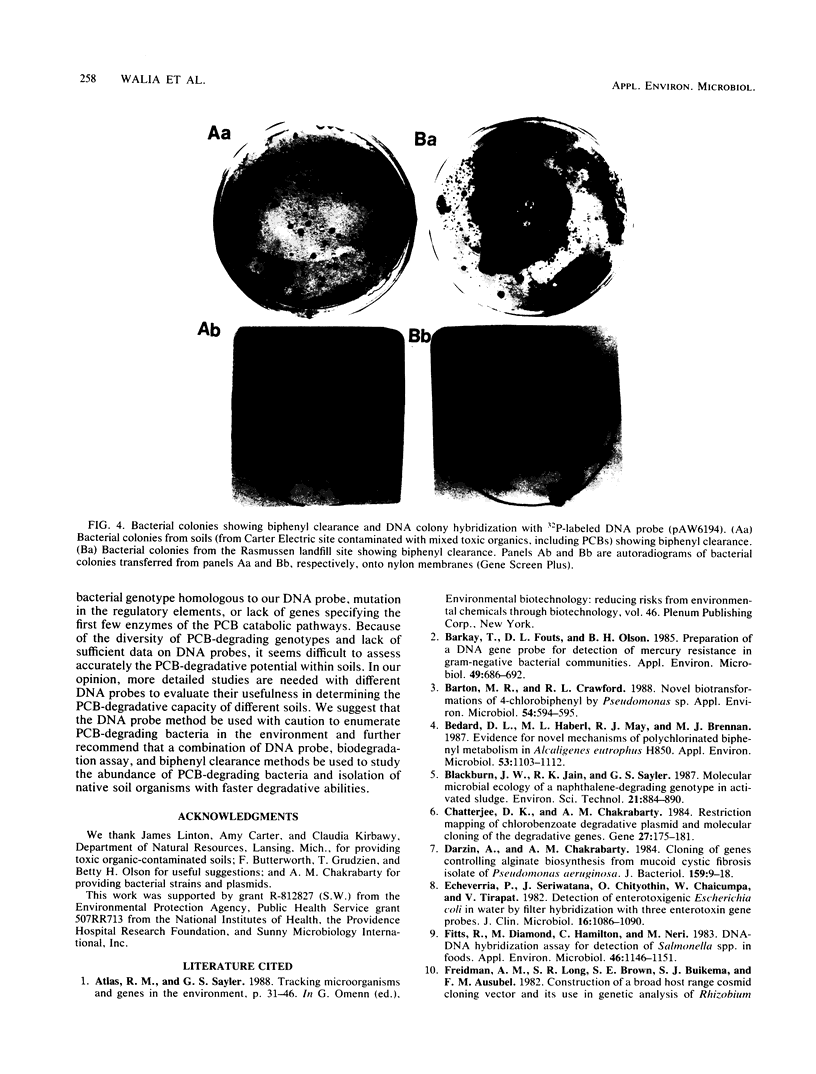
Several DNA probes for polychlorinated biphenyl (PCB)-degrading genotypes were constructed from PCB-degrading bacteria. These laboratory-engineered DNA probes were used for the detection, enumeration, and isolation of specific bacteria degrading PCBs. Dot blot analysis of purified DNA from toxic organic chemical-contaminated soil bacterial communities showed positive DNA-DNA hybridization with a 32P-labeled DNA probe (pAW6194, cbpABCD). Less than 1% of bacterial colonies isolated from garden topsoil and greater than 80% of bacteria isolated from PCB-contaminated soils showed DNA homologies with 32P-labeled DNA probes. Some of the PCB-degrading bacterial isolates detected by the DNA probe method did not show biphenyl clearance. The DNA probe method was found to detect additional organisms with greater genetic potential to degrade PCBs than the biphenyl clearance method did. Results from this study demonstrate the usefulness of DNA probes in detecting specific PCB-degrading bacteria, abundance of PCB-degrading genotypes, and genotypic diversity among PCB-degrading bacteria in toxic chemical-polluted soil environments. We suggest that the DNA probe should be used with caution for accurate assessment of PCB-degradative capacity within soils and further recommend that a combination of DNA probe and biodegradation assay be used to determine the abundance of PCB-degrading bacteria in the soil bacterial community.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkay T., Fouts D. L., Olson B. H. Preparation of a DNA gene probe for detection of mercury resistance genes in gram-negative bacterial communities. Appl Environ Microbiol. 1985 Mar;49(3):686–692. doi: 10.1128/aem.49.3.686-692.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M. R., Crawford R. L. Novel biotransformations of 4-chlorobiphenyl by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):594–595. doi: 10.1128/aem.54.2.594-595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Haberl M. L., May R. J., Brennan M. J. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1103–1112. doi: 10.1128/aem.53.5.1103-1112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D. K., Chakrabarty A. M. Restriction mapping of a chlorobenzoate degradative plasmid and molecular cloning of the degradative genes. Gene. 1984 Feb;27(2):173–181. doi: 10.1016/0378-1119(84)90138-0. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria P., Seriwatana J., Chityothin O., Chaicumpa W., Tirapat C. Detection of enterotoxigenic Escherichia coli in water by filter hybridization with three enterotoxin gene probes. J Clin Microbiol. 1982 Dec;16(6):1086–1090. doi: 10.1128/jcm.16.6.1086-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R., Diamond M., Hamilton C., Neri M. DNA-DNA hybridization assay for detection of Salmonella spp. in foods. Appl Environ Microbiol. 1983 Nov;46(5):1146–1151. doi: 10.1128/aem.46.5.1146-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Arimura N. Purification and properties of 2,3-dihydroxybiphenyl dioxygenase from polychlorinated biphenyl-degrading Pseudomonas pseudoalcaligenes and Pseudomonas aeruginosa carrying the cloned bphC gene. J Bacteriol. 1987 Feb;169(2):924–927. doi: 10.1128/jb.169.2.924-927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Payne W. L., Aulisio C. C. Detection and enumeration of virulent Yersinia enterocolitica in food by DNA colony hybridization. Appl Environ Microbiol. 1983 Sep;46(3):636–641. doi: 10.1128/aem.46.3.636-641.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagow J., Hill W. E. Enumeration by DNA colony hybridization of virulent Yersinia enterocolitica colonies in artificially contaminated food. Appl Environ Microbiol. 1986 Feb;51(2):441–443. doi: 10.1128/aem.51.2.441-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K., Sayler G. S., Wilson J. T., Houston L., Pacia D. Maintenance and stability of introduced genotypes in groundwater aquifer material. Appl Environ Microbiol. 1987 May;53(5):996–1002. doi: 10.1128/aem.53.5.996-1002.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil H., Lebens M. R., Williams P. A. TOL plasmid pWW15 contains two nonhomologous, independently regulated catechol 2,3-oxygenase genes. J Bacteriol. 1985 Jul;163(1):248–255. doi: 10.1128/jb.163.1.248-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Tewari R., Walia S. Molecular cloning of 3-phenylcatechol dioxygenase involved in the catabolic pathway of chlorinated biphenyl from Pseudomonas putida and its expression in Escherichia coli. Appl Environ Microbiol. 1988 Nov;54(11):2664–2671. doi: 10.1128/aem.54.11.2664-2671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Walia S. Cloning of bacterial genes specifying degradation of 4-chlorobiphenyl from Pseudomonas putida OU83. Appl Environ Microbiol. 1989 Apr;55(4):798–805. doi: 10.1128/aem.55.4.798-805.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Xu J. G., Kaper J. B., Lior H., Prado V., Tall B., Nataro J., Karch H., Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987 Jul;156(1):175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- Petrick H. A., Ambrosio R. E., Holzapfel W. H. Isolation of a DNA Probe for Lactobacillus curvatus. Appl Environ Microbiol. 1988 Feb;54(2):405–408. doi: 10.1128/aem.54.2.405-408.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin F. A., Kopecko D. J., Noon K. F., Baron L. S. Development of a DNA probe to detect Salmonella typhi. J Clin Microbiol. 1985 Oct;22(4):600–605. doi: 10.1128/jcm.22.4.600-605.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangodkar U. M., Chapman P. J., Chakrabarty A. M. Cloning, physical mapping and expression of chromosomal genes specifying degradation of the herbicide 2,4,5-T by Pseudomonas cepacia AC1100. Gene. 1988 Nov 30;71(2):267–277. doi: 10.1016/0378-1119(88)90043-1. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Shields M. S., Tedford E. T., Breen A., Hooper S. W., Sirotkin K. M., Davis J. W. Application of DNA-DNA colony hybridization to the detection of catabolic genotypes in environmental samples. Appl Environ Microbiol. 1985 May;49(5):1295–1303. doi: 10.1128/aem.49.5.1295-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethabutr O., Hanchalay S., Echeverria P., Taylor D. N., Leksomboon U. A non-radioactive DNA probe to identify Shigella and enteroinvasive Escherichia coli in stools of children with diarrhoea. Lancet. 1985 Nov 16;2(8464):1095–1097. doi: 10.1016/s0140-6736(85)90687-7. [DOI] [PubMed] [Google Scholar]
- Simonet P., Le N. T., Du Cros E. T., Bardin R. Identification of frankia strains by direct DNA hybridization of crushed nodules. Appl Environ Microbiol. 1988 Oct;54(10):2500–2503. doi: 10.1128/aem.54.10.2500-2503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taira K., Hayase N., Arimura N., Yamashita S., Miyazaki T., Furukawa K. Cloning and nucleotide sequence of the 2,3-dihydroxybiphenyl dioxygenase gene from the PCB-degrading strain of Pseudomonas paucimobilis Q1. Biochemistry. 1988 May 31;27(11):3990–3996. doi: 10.1021/bi00411a015. [DOI] [PubMed] [Google Scholar]
- Walia S. K., Duckworth D. H. The relationship of the delta transfer factor and KColIb to the ColIb plasmid. J Gen Microbiol. 1986 Dec;132(12):3261–3268. doi: 10.1099/00221287-132-12-3261. [DOI] [PubMed] [Google Scholar]
- Wood P. K., Morris J. G., Jr, Small P. L., Sethabutr O., Toledo M. R., Trabulsi L., Kaper J. B. Comparison of DNA probes and the Sereny test for identification of invasive Shigella and Escherichia coli strains. J Clin Microbiol. 1986 Sep;24(3):498–500. doi: 10.1128/jcm.24.3.498-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R., Mondello F. J. Sequence similarities in the genes encoding polychlorinated biphenyl degradation by Pseudomonas strain LB400 and Alcaligenes eutrophus H850. J Bacteriol. 1989 Mar;171(3):1733–1735. doi: 10.1128/jb.171.3.1733-1735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeph L. R., Stotzky G. Use of a biotinylated DNA probe to detect bacteria transduced by bacteriophage P1 in soil. Appl Environ Microbiol. 1989 Mar;55(3):661–665. doi: 10.1128/aem.55.3.661-665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]