Abstract
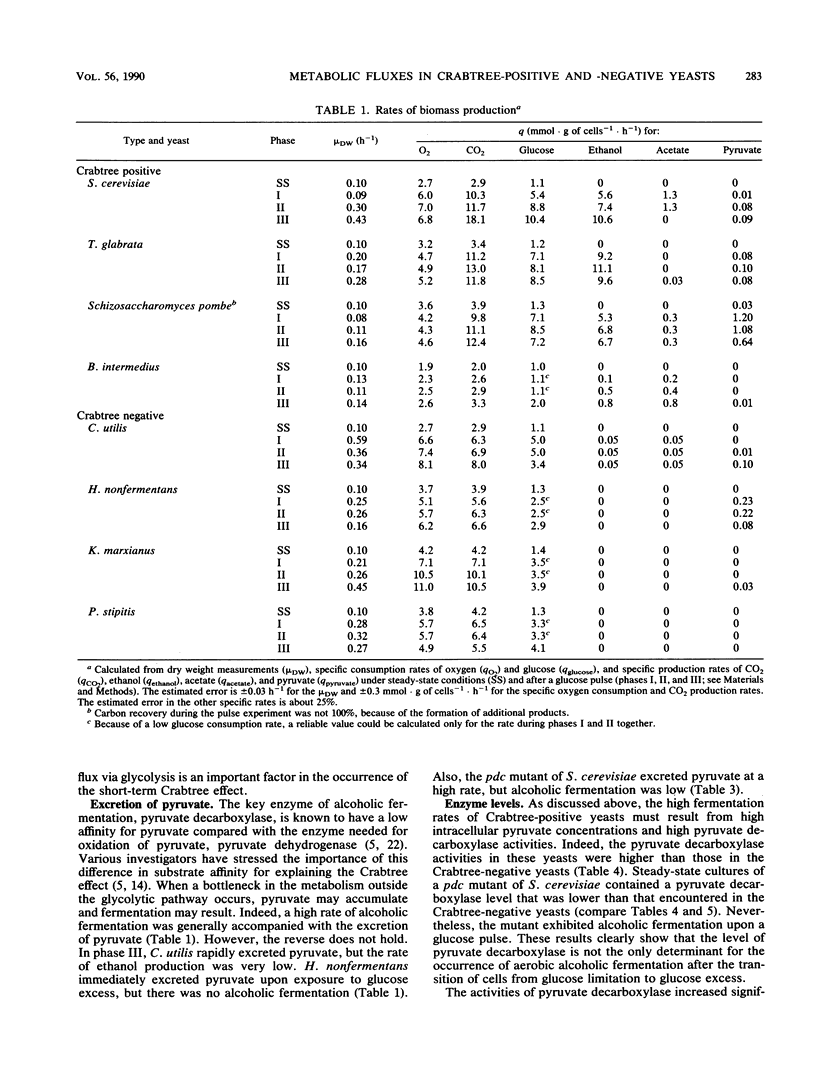
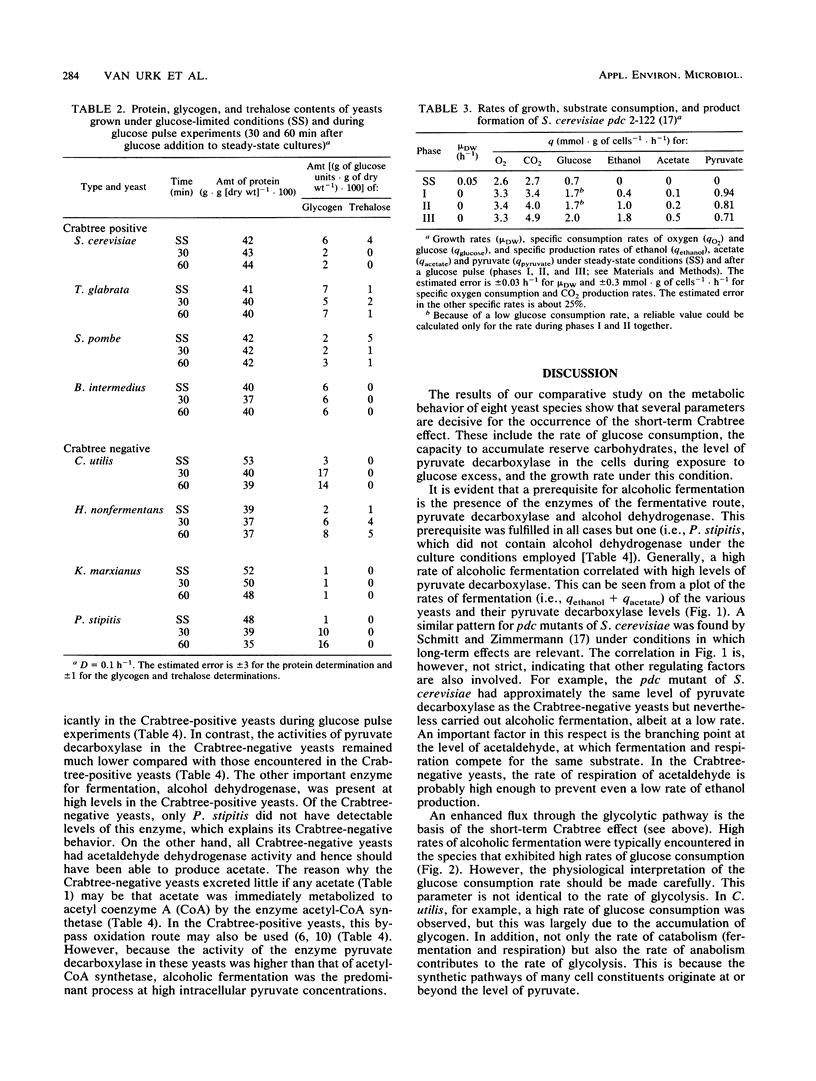
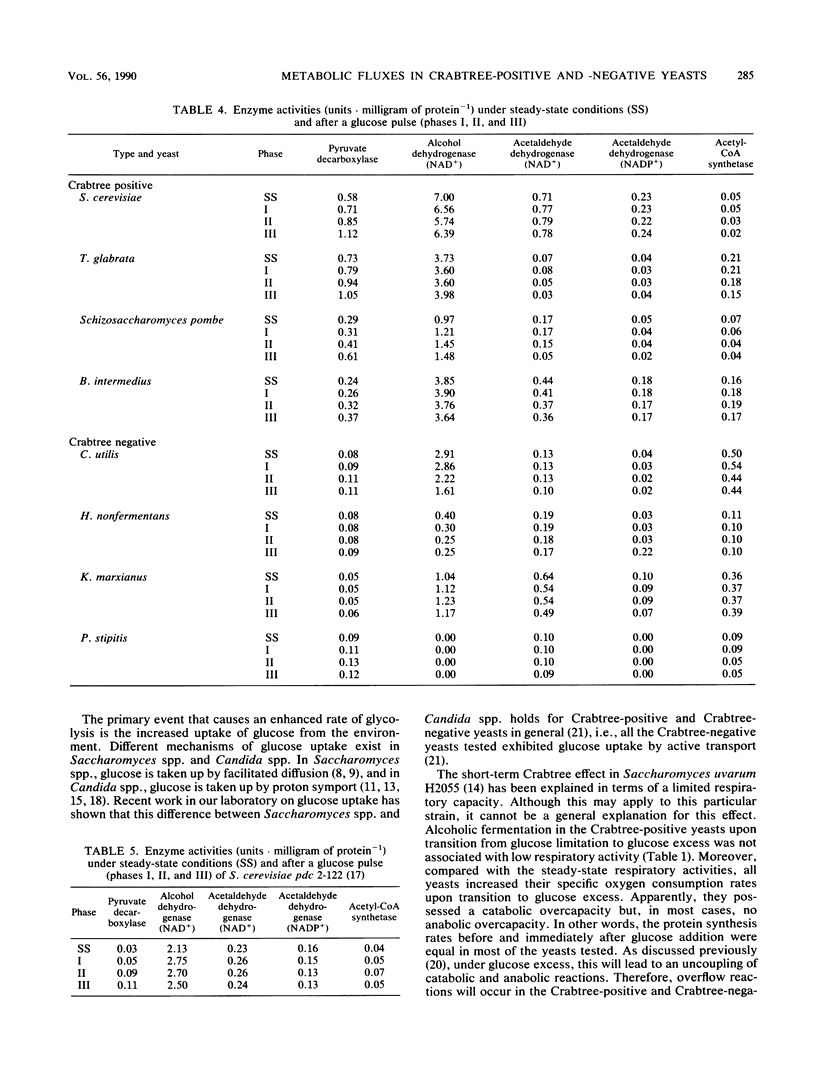
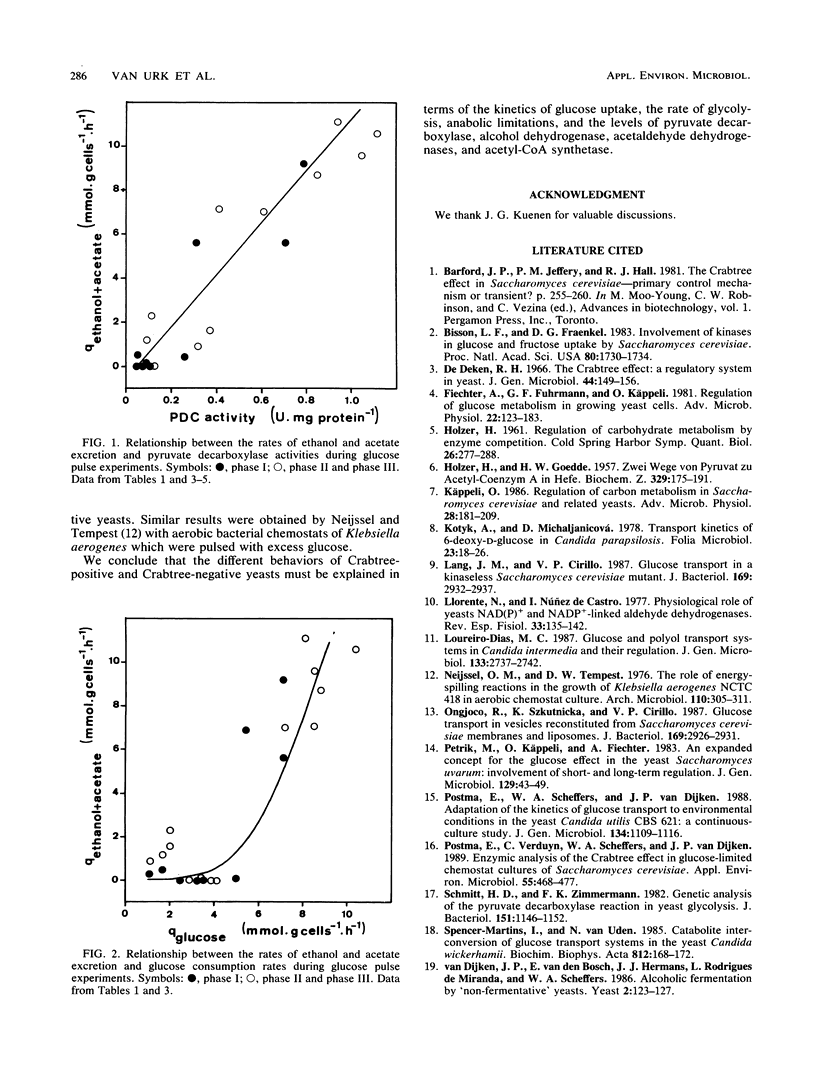
In bakers' yeast, an immediate alcoholic fermentation begins when a glucose pulse is added to glucose-limited, aerobically grown cells. The mechanism of this short-term Crabtree effect was investigated via a comparative enzymic analysis of eight yeast species. It was established that the fermentation rate of the organisms upon transition from glucose limitation to glucose excess is positively correlated with the level of pyruvate decarboxylase (EC 4.1.1.1). In the Crabtree-negative yeasts, the pyruvate decarboxylase activity was low and did not increase when excess glucose was added. In contrast, in the Crabtree-positive yeasts, the activity of this enzyme was on the average sixfold higher and increased after exposure to glucose excess. In Crabtree-negative species, relatively high activities of acetaldehyde dehydrogenases (EC 1.2.1.4 and EC 1.2.1.5) and acetyl coenzyme A synthetase (EC 6.2.1.1), in addition to low pyruvate decarboxylase activities, were present. Thus, in these yeasts, acetaldehyde can be effectively oxidized via a bypass that circumvents the reduction of acetaldehyde to ethanol. Growth rates of most Crabtree-positive yeasts did not increase upon transition from glucose limitation to glucose excess. In contrast, the Crabtree-negative yeasts exhibited enhanced rates of biomass production which in most cases could be ascribed to the intracellular accumulation of reserve carbohydrates. Generally, the glucose consumption rate after a glucose pulse was higher in the Crabtree-positive yeasts than in the Crabtree-negative yeasts. However, the respiratory capacities of steady-state cultures of Crabtree-positive yeasts were not significantly different from those of Crabtree-negative yeasts. Thus, a limited respiratory capacity is not the primary cause of the Crabtree effect in yeasts. Instead, the difference between Crabtree-positive and Crabtree-negative yeasts is attributed to differences in the kinetics of glucose uptake, synthesis of reserve carbohydrates, and pyruvate metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effect: a regulatory system in yeast. J Gen Microbiol. 1966 Aug;44(2):149–156. doi: 10.1099/00221287-44-2-149. [DOI] [PubMed] [Google Scholar]
- Fiechter A., Fuhrmann G. F., Käppeli O. Regulation of glucose metabolism in growing yeast cells. Adv Microb Physiol. 1981;22:123–183. doi: 10.1016/s0065-2911(08)60327-6. [DOI] [PubMed] [Google Scholar]
- HOLZER H., GOEDDE H. W. Zwei Wege von Pyruvat zu Acetyl-Coenzym A in Hefe. Biochem Z. 1957;329(3):175–191. [PubMed] [Google Scholar]
- HOLZER H. Regulation of carbohydrate metabolism by enzyme competition. Cold Spring Harb Symp Quant Biol. 1961;26:277–288. doi: 10.1101/sqb.1961.026.01.034. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Michaljanicová D. Transport kinetics of 6-deoxy-D-glucose in Candida parapsilosis. Folia Microbiol (Praha) 1978;23(1):18–26. doi: 10.1007/BF02876591. [DOI] [PubMed] [Google Scholar]
- Käppeli O. Regulation of carbon metabolism in Saccharomyces cerevisiae and related yeasts. Adv Microb Physiol. 1986;28:181–209. doi: 10.1016/s0065-2911(08)60239-8. [DOI] [PubMed] [Google Scholar]
- Lang J. M., Cirillo V. P. Glucose transport in a kinaseless Saccharomyces cerevisiae mutant. J Bacteriol. 1987 Jul;169(7):2932–2937. doi: 10.1128/jb.169.7.2932-2937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente N., de Castro I. N. Physiological role of yeasts NAD(P)+ and NADP+-linked aldehyde dehydrogenases. Rev Esp Fisiol. 1977 Jun;33(2):135–142. [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. The role of energy-spilling reactions in the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat culture. Arch Microbiol. 1976 Nov 2;110(23):305–311. doi: 10.1007/BF00690243. [DOI] [PubMed] [Google Scholar]
- Ongjoco R., Szkutnicka K., Cirillo V. P. Glucose transport in vesicles reconstituted from Saccharomyces cerevisiae membranes and liposomes. J Bacteriol. 1987 Jul;169(7):2926–2931. doi: 10.1128/jb.169.7.2926-2931.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E., Verduyn C., Scheffers W. A., Van Dijken J. P. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989 Feb;55(2):468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H. D., Zimmermann F. K. Genetic analysis of the pyruvate decarboxylase reaction in yeast glycolysis. J Bacteriol. 1982 Sep;151(3):1146–1152. doi: 10.1128/jb.151.3.1146-1152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Urk H., Bruinenberg P. M., Veenhuis M., Scheffers W. A., Van Dijken J. P. Respiratory capacities of mitochondria of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 grown under glucose limitation. Antonie Van Leeuwenhoek. 1989 Oct;56(3):211–220. doi: 10.1007/BF00418933. [DOI] [PubMed] [Google Scholar]
- Van Urk H., Mak P. R., Scheffers W. A., van Dijken J. P. Metabolic responses of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 upon transition from glucose limitation to glucose excess. Yeast. 1988 Dec;4(4):283–291. doi: 10.1002/yea.320040406. [DOI] [PubMed] [Google Scholar]
- van Urk H., Postma E., Scheffers W. A., van Dijken J. P. Glucose transport in crabtree-positive and crabtree-negative yeasts. J Gen Microbiol. 1989 Sep;135(9):2399–2406. doi: 10.1099/00221287-135-9-2399. [DOI] [PubMed] [Google Scholar]
- van Urk H., Schipper D., Breedveld G. J., Mak P. R., Scheffers W. A., van Dijken J. P. Localization and kinetics of pyruvate-metabolizing enzymes in relation to aerobic alcoholic fermentation in Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621. Biochim Biophys Acta. 1989 Jul 21;992(1):78–86. doi: 10.1016/0304-4165(89)90053-6. [DOI] [PubMed] [Google Scholar]


