Abstract
Functional activity of N-methyl-d-aspartate (NMDA) receptors requires both glutamate binding and the binding of an endogenous coagonist that has been presumed to be glycine, although d-serine is a more potent agonist. Localizations of d-serine and it biosynthetic enzyme serine racemase approximate the distribution of NMDA receptors more closely than glycine. We now show that selective degradation of d-serine with d-amino acid oxidase greatly attenuates NMDA receptor-mediated neurotransmission as assessed by using whole-cell patch–clamp recordings or indirectly by using biochemical assays of the sequelae of NMDA receptor-mediated calcium flux. The inhibitory effects of the enzyme are fully reversed by exogenously applied d-serine, which by itself did not potentiate NMDA receptor-mediated synaptic responses. Thus, d-serine is an endogenous modulator of the glycine site of NMDA receptors and fully occupies this site at some functional synapses.
D-Amino acids play prominent roles in bacteria but have not been thought to occur in substantial quantity or to have any important function in vertebrates. Recently, techniques to distinguish isomers of amino acids in routine assays have led to the identification in some mammalian tissues of substantial amounts of at least two d-amino acids, d-serine and d-aspartate (1). Although d-aspartate is present in selected neuronal populations in the brain, it is concentrated mainly in glands, especially the epinephrine-containing cells of the adrenal medulla, the posterior pituitary, and the pineal gland (2–4).
In contrast, d-serine occurs primarily in the brain, with highest concentrations in regions enriched in N-methyl-d-aspartate (NMDA) receptors (5–7). In these areas immunohistochemical studies have localized d-serine to protoplasmic astrocytes, which ensheathe nerve terminals especially in areas of the brain enriched in NMDA receptors (7). Stimulation of the kainate subtype of glutamate receptor releases d-serine from protoplasmic astrocytes (7).
Because exogenous d-serine potentiates NMDA receptor-mediated neurotransmission (8–11) and d-[3H]serine selectively binds to the glycine site (6), d-serine has been proposed as an endogenous ligand for the strychnine-insensitive glycine site of the NMDA receptor (6). Activation of NMDA receptors requires the presence of a coagonist, initially thought to be glycine (8, 12–14), and a glycine-selective recognition domain has been localized on NMDA receptors (15–17). However, d-serine is at least as potent as glycine as a coagonist at this site (8, 10, 14). In addition, immunohistochemical studies have revealed an overlapping distribution of d-serine and NMDA receptor immunoreactivity in forebrain (7). In the developing cerebellum, d-serine is localized to Bergmann glia that regulate granule cell migration during development via NMDA receptors (7). In contrast, glycine immunoreactivity is localized differently from that of NMDA receptors except in the brainstem, where it closely parallels the distribution of NMDA receptors (7). Extracellular levels of d-serine in the forebrain are comparable to glycine, whereas in some areas, such as striatum, extracellular d-serine concentration is 2.6-fold higher than glycine (18).
The localization of d-serine to glia in the vicinity of neurons enriched in NMDA receptors, the release of endogenous d-serine by kainate, and the ability of exogenous d-serine to activate the glycine site of NMDA receptors all suggest that d-serine is an endogenous ligand for the glycine site. However, until now, there has been no direct evidence for such a role. In the present study, we have monitored NMDA receptor-mediated neurotransmission electrophysiologically as well as by the stimulation of NO synthase activity and cGMP levels. We demonstrate that NMDA receptor-mediated synaptic transmission is diminished greatly by treatment with d-amino acid oxidase (DAAOX), which depletes endogenous d-serine but not glycine.
Experimental Procedures
Materials.
d-Amino acid oxidase (DAAOX) was purchased from Boehringer Mannheim in suspension with 3.2 M ammonium sulfate solution, pH 6.5. Each new batch of DAAOX was dialyzed for 8 h at 4°C against 20 mM phosphate buffer (pH 7.4) with two to three changes and stored at −20°C with no detectable decrease in activity. SCH50911 was obtained from Tocris Neuramin (Bristol, U.K.). 2,3-Dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) and d-serine were purchased from Research Biochemicals. Cs-BAPTA and QX-314 were obtained from Molecular Probes and Alomone (Jerusalem), respectively. 3-[(±)-2-Carboxypiperazin-4-yl]propyl-1-phosphonate (CPP) was a generous gift from Sandoz Pharmaceutical. N-tert-butyloxycarbonyl-l-cysteine was purchased from Nova Biochem. All other compounds were obtained from Sigma.
Animals.
Biochemical experiments were performed on cerebelli removed from male postnatal day 9–11 or 6-week-old Sprague–Dawley rats. For electrophysiological experiments, hippocampi from postnatal day 13–16 male Sprague–Dawley rats (slice recording) or from 19-day-old rat embryos (cell cultures) were used. Rats were housed under a 12-h light/12-h dark cycle with food and water ad libitum. All animal-use procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Biochemical Assays.
Protein concentrations were assayed by using the Coomassie Plus reagent (Pierce). Each new batch of DAAOX was assayed for the presence of any contaminant by SDS/PAGE gel (12% acrylamide) followed by Coomassie staining. DAAOX was assayed by a chemiluminescent assay method for evolved H2O2 (19). NO synthase was assayed by monitoring the conversion of [3H]l-arginine to [3H]l-citrulline (20). cGMP was monitored by an RIA kit (Amersham Pharmacia). To measure amino acid enantiomers, brain slices, cultured cells, and culture media were homogenized at 4°C in 1 M trichloroacetic acid (TCA) and the homogenate was centrifuged at 10,000 × g for 15 min at 4°C. TCA was extracted three times with 2 ml of water-saturated diethyl ether, and the aqueous layer was stored at −80°C. Detection of free d- and l-amino acids was performed by fluorescence HPLC after derivatization with N-tert-butyloxycarbonyl-l-cysteine/o-phthaldialdehyde as described (21).
Electrophysiology.
Rats were anesthetized lightly with halothane and decapitated, and the hippocampi were dissected quickly. Transverse slices (500-μm thick) were cut by using a Vibratome in oxygenated (95% O2/5% CO2), ice-cold, Ca2+-free artificial cerebrospinal fluid. The slices were held at room temperature for at least 60 min in standard oxygenated artificial cerebrospinal fluid (aCSF) containing 3 mM CaCl2 before transfer to an interface-type recording chamber perfused with oxygenated, Ca2+-containing aCSF (1 ml/min) and were maintained at 33°C. The slices were allowed to equilibrate for an additional 60 min in the recording chamber. Catalase (1,000 units/ml) was added to the aCSF to degrade the peroxide products produced by metabolism of d-serine. Field excitatory postsynaptic potentials were elicited by alternately delivering 0.05-msec constant-current pulses at 0.033 Hz through two platinum concentric bipolar stimulation electrodes placed on opposite sides of the recording site in the stratum radiatum of the CA1 region. The resulting extracellular potentials were monitored by using a low-resistance (1–5 MΩ) glass pipette filled with 1 M NaCl. The stimulus intensity then was adjusted to evoke a test response that was approximately 30% of the maximal level. Stable baseline field responses were recorded for at least 60 min before beginning the experiment. The area of the synaptic response was determined and used to calculate the percent change with respect to the area at zero time.
Whole-cell recordings were obtained from pyramidal-shaped neurons in mixed hippocampal neuron–astrocyte cultures prepared from 19-day-old rat embryos and maintained in vitro for 12–15 days (22). During recording, the cells were perfused continuously (0.5 ml/min) with an external solution of Hepes buffer (10 mM) containing NaCl (140 mM), KCl (5 mM), CaCl2 (3 mM), dextrose (10 mM), NBQX (10 μM), picrotoxin (50 μM), strychnine (5 μM), SCH50911 (10 μM), and catalase (500 units/ml) adjusted to pH 7.4. To record α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated synaptic currents, CPP (50 μM) was substituted for NBQX and the recording solution was supplemented with MgCl2 (1.5 mM). In experiments examining responses to exogenously applied NMDA, tetrodotoxin (1 μM) was included in the perfusion solution.
Patch electrodes (3–5 MΩ) were filled with solution containing cesium gluconate (100 mM), Hepes (40 mM), MgCl2 (5 mM), cesium-BAPTA (10 mM), Na2-ATP (5 mM), Na3-GTP (0.5 mM), and lidocaine N-ethyl bromide (QX-314; 5 mM). Whole-cell currents were recorded from individual pyramidal cells under voltage–clamp at room temperature. Cells were held at −70 mV. The currents were filtered at 2 kHz and digitized at 5 kHz.
Test substances were dissolved in external solution and were applied by using a switched multibarrel gravity-fed perfusion system in which the perfusion solution exited via two barrels positioned close to the cell of interest. Unless otherwise indicated, test solutions were applied for periods of 5 min after a 20- to 30-min baseline recording period. In some experiments, NMDA-evoked synaptic responses were simulated with puffs of NMDA solution (100 μM) applied by air-pressure ejection (5–15 psi; 1 psi = 6.89 kPa) from a glass micropipette situated near the cell under study. In these experiments, other test solutions were applied by using the gravity-fed multibarrel perfusion system. In other experiments, NMDA receptor currents were evoked with the multibarrel perfusion system, in which case the NMDA concentration was 10 μM.
Statistical Analysis.
Data were analyzed by one-way ANOVA with independent posthoc Turkey's or Scheffé multiple comparison test except for analysis of HPLC data, in which case a Student's t test was used. Data are presented as mean ± SEM.
Results
DAAOX is a highly selective enzyme that degrades only neutral d-amino acids (23, 24). Its best substrates in vitro are d-serine and d-alanine. Glycine is not a substrate at physiological pH values, although modest activity toward glycine is evident at pH 10. The purity of DAAOX was verified as a single, 39-kDa band on SDS/PAGE. The enzyme exhibited robust degradation in pure solution of d-serine but not glycine or NMDA (data not shown). To characterize further the specificity of DAAOX, we evaluated the influence of the enzyme on endogenous amino acid concentrations in cerebellar slices of immature rats (postnatal day 9–11), where d-serine concentrations are high (5–7). As shown in Table 1, glutamate and glutamine levels are highest, whereas d-serine levels are 15% of l-serine levels and 40% of the levels of glycine. d-aspartate levels are only about 4% of those of l-aspartate. DAAOX treatment reduces d-serine levels by 90% while exerting no influence on levels of glycine, d-aspartate, or any other amino acid assayed. Thus, DAAOX acts highly selectively on d-serine under our experimental conditions.
Table 1.
DAAOX selectively depletes d-serine in cerebellar slices
| Amino acid | Content, nmol/mg protein
|
Effect of DAAOX, % of control | |
|---|---|---|---|
| Before DAAOX | After DAAOX | ||
| l-serine | 7.2 ± 0.3 | 8.1 ± 0.6 | 112.5 |
| d-serine | 1.1 ± 0.20 | 0.12 ± 0.03 | 10.9* |
| Glycine | 2.8 ± 0.7 | 2.7 ± 0.4 | 96.4 |
| l-Glutamate | 25.5 ± 3.0 | 28.5 ± 4.5 | 111.8 |
| l-Glutamate | 18.7 ± 1.6 | 19.7 ± 1.8 | 105.3 |
| l-Aspartate | 9.6 ± 1.4 | 10.9 ± 2.0 | 113.5 |
| d-Aspartate | 0.35 ± 0.07 | 0.33 ± 0.05 | 94.3 |
Separation of free d- and l-amino acids was performed by HPLC as described in Experimental Procedures. Content values are expressed as mean values ± SEM for five independent determinations.
*P = 0.0095, t test.
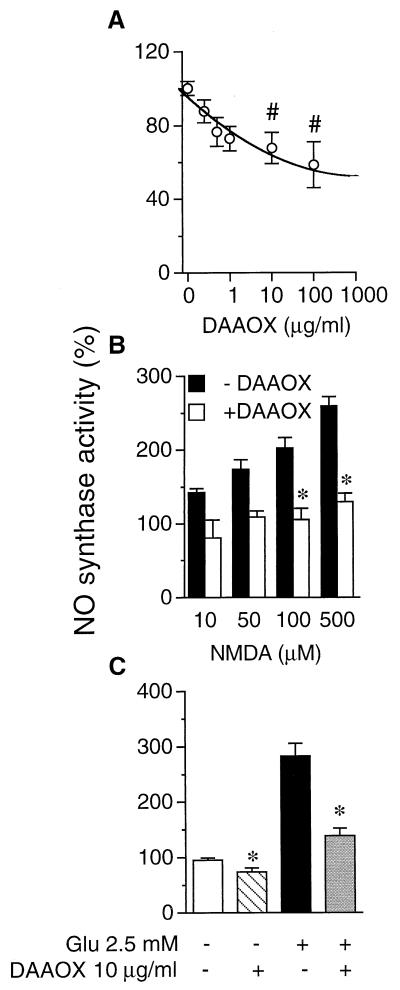
In cerebellar slices from immature rats, NMDA rapidly and markedly augments the activity of neuronal NO synthase (NOS) (25, 26), which we confirm here (Fig. 1). Addition of purified DAAOX in cerebellar slices diminishes basal NOS activity in cerebellar slices maximally about 50% at 100 μg/ml DAAOX (Fig. 1A). Heat-inactivated DAAOX fails to produce any significant effect on NOS activity (data not shown). The reduction of NOS activity in the absence of NMDA indicates that basal NOS activity is regulated by endogenous d-serine. We further investigated the effects of DAAOX on NMDA-evoked NOS activity. NMDA (10–500 μM) stimulates basal NOS activity 50–250%, and DAAOX abolishes the stimulation, reducing NMDA evoked NOS activity to basal levels (Fig. 1B). The proportional decrease of NOS activity is fairly similar over a wide range of NMDA concentrations. Like NMDA, glutamate stimulates NOS activity 2.8-fold and DAAOX prevents this stimulation (Fig. 1C).
Figure 1.
DAAOX diminishes basal and evoked cerebellar NMDA receptor-mediated neurotransmission monitored by NOS activation. The activity of NOS was used as an index of NMDA receptor activity. NOS was assayed by the conversion of [3H]l-arginine (3 μCi/ml in the assay) to [3H]l-citrulline in cerebellar slices from 9- to 11-day-old rats. (A) DAAOX reduces basal NOS activity. The IC50 for DAAOX is ≈10 μg/ml. (B) NMDA augments NOS activity in a concentration-dependent manner. DAAOX (10 μg/ml) diminishes NMDA-evoked NOS activity. When DAAOX is heat-inactivated, it fails to reduce basal or NMDA-evoked NOS activity (data not shown). (C) DAAOX (10 μg/ml) reduces glutamate-evoked NOS activity. Data are the mean ± SEM of three to five independent experiments, with triplicate determinations in each case. The basal activity was 5,300 ± 603 cpm/mg. Statistical comparisons are with respect to the corresponding condition in the absence of DAAOX. #, P < 0.05 and *, P < 0.001; one-way ANOVA with Tukey's multiple comparison test.
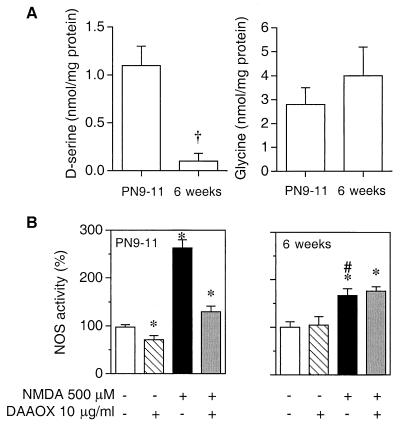
NMDA stimulation of NOS activity and of cGMP levels in cerebellar slices is greater in immature than adult cerebellum (25, 26). Previously, we reported that d-serine in the cerebellum declines to negligible levels in the adult (7). In the present study, using HPLC, we confirm the substantial levels of d-serine in cerebellar slices of 9- to 11-day-old rats, whereas d-serine is virtually undetectable in the cerebellum of 6-week-old rats (Fig. 2A). As described previously (25), NMDA stimulation of NOS activity in adult cerebellar slices is significantly less than in slices from immature rats (Fig. 2B). This difference is not due to changes in the pattern of expression of NOS, because immunoblotting analysis reveals no decrease in the level of the protein between immature and adult rats (data not shown), and it cannot be accounted for by changes in the levels of glycine (Fig. 2A). In marked contrast to findings in the immature rats, in adult tissue, DAAOX fails to reduce either basal or NMDA-stimulated NOS activity (Fig. 2B). Coupled with the absence of detectable d-serine in adult cerebellum, these findings indicate that regulation of NMDA receptor activity by endogenous d-serine in immature cerebellum no longer takes place in the adult. In addition, this latter observation weighs against the possibility that DAAOX itself interferes with the function of NMDA receptors and confirms that d-serine is not a contaminant of the solutions used in our experiments as demonstrated by HPLC analysis (see below).
Figure 2.
DAAOX does not affect either basal or NMDA-evoked NOS activity monitored in mature cerebellum. (A) d-Serine and glycine levels were determined by HPLC in cerebellar slices from immature (9- to 11-day-old) and adult (6-week-old) rats. †, P < 0.0001; t test. (B) Basal and NMDA-evoked NOS activity was determined as in Fig. 1. Data are the means ± SEM of four independent experiments, with triplicate determinations in each case. Statistical comparisons are with respect to the corresponding controls in the absence of NMDA and DAAOX. *, P < 0.001; one-way ANOVA with Tukey's multiple comparison test. NMDA-evoked NOS activity in adult was significantly less than in the immature cerebellum (#, P < 0.05).
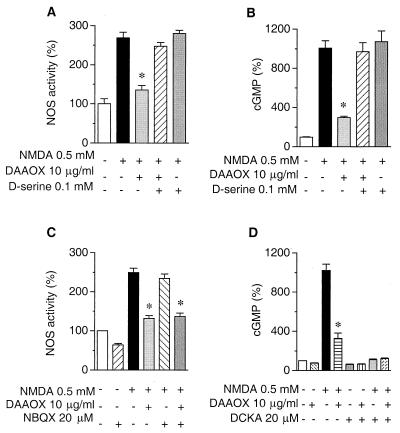
Interpreting the influence of DAAOX on NMDA receptor function depends on the selectivity of enzyme action. The catalytic degradation of d-serine by DAAOX generates hydrogen peroxide (19) that could cause lipid peroxidation, which can irreversibly alter NMDA receptor function (27). To avoid this possibility, excess catalase was included in all incubation solutions. Moreover, in studies on cerebellar slices, the enzyme preparation did not exhibit any proteolytic activity that could degrade NMDA receptors (data not shown). To directly demonstrate the functional integrity of NMDA receptors, exogenous d-serine was used to reverse the DAAOX effect. d-Serine (0.1 mM) fully reverses the inhibitory effect of DAAOX on NOS activity (Fig. 3A), and glycine (0.1 mM) has a similar action (data not shown). In the absence of DAAOX, the increase of NOS activity elicited by NMDA is the same in the presence or absence of d-serine (Fig. 3A). DAAOX treatment reduces NOS activity to levels almost as low as in the absence of NMDA. d-Serine fully reverses these effects.
Figure 3.
d-Serine reverses inhibitory effects of DAAOX on NMDA receptor activation in cerebellar slices. NOS was monitored as in Fig. 1, and cGMP formation was measured by RIA. (A and B) The inhibitory effect of DAAOX on NMDA-evoked NOS activity (A) and cGMP formation (B) was almost wholly reversed by exogenous d-serine. (C) The AMPA/kainate receptor antagonist NBQX does not influence the inhibitory action of DAAOX on NMDA-evoked NOS activity. (D) Effects of 5,7-dichlorokynurenic acid (DCKA), an antagonist of the strychnine-insensitive glycine site, on NMDA-stimulated cGMP formation. Data are the means ± SEM of three to five independent experiments, with triplicate determinations in each case. Some of the SE bars are too small to be seen at this scale. Asterisks indicate a significant difference from NMDA-stimulated values. *, P < 0.001; one-way ANOVA with Tukey's multiple comparison test.
We also monitored effects of DAAOX on NMDA stimulation of cGMP levels in cerebellar slices from immature rats (Fig. 3B). NMDA elicits an 8- to 10-fold stimulation of cGMP levels, substantially greater than the augmentation of NOS activity, which is the same with or without added d-serine (0.1 mM). DAAOX reduces the influence of NMDA by 71 ± 2%. The actions of DAAOX on both NOS activity and cGMP are reversed completely by d-serine, indicating that DAAOX effects do not reflect nonspecific proteolysis of NMDA receptors by a contaminating enzyme or irreversible receptor oxidation by hydrogen peroxide generated from DAAOX activity.
To ensure the selectivity of DAAOX for NMDA receptors, we used various receptor antagonists. To evaluate AMPA and/or kainate receptors, we used NBQX, a selective AMPA and kainate receptor antagonist that does not interact with the glycine site of NMDA receptors (28–30). NBQX (20 μM) does not influence NMDA-evoked NOS activation in the absence or presence of DAAOX whereas it decreases by 36% the basal activity of NOS when applied alone (Fig. 3C). This latter observation shows that 20 μM NBQX blocks AMPA and kainate receptor-mediated NOS activation so that some fraction of basal NOS activity is dependent on these receptors. 5,7-Dichlorokynurenic acid, an antagonist of the glycine site of NMDA receptors (31, 32), reduces by 33% the basal level of cGMP in cerebellar slices (Fig. 3D) and completely blocks the increase in cGMP induced by NMDA. In these experiments, DAAOX reduces by 76% the NMDA stimulation of cGMP levels. 5,7-Dichlorokynurenic acid virtually abolishes NMDA-induced cGMP production, and DAAOX does not exert any additional effect. As expected, MK-801 (20 μM) blocks NMDA-induced cGMP formation and NOS activation (data not shown).
We carried out experiments by using hippocampal slices to determine the role of d-serine as a modulator of physiologically activated NMDA receptors. In slices perfused with a recording solution containing 20 μM NBQX, 100 μM picrotoxin, 20 μM SCH50911, and 10 μM strychnine to block AMPA/kainate, γ-aminobutyric acid type A (GABAA), GABAB, and glycine receptor currents, respectively, CA1 field responses [field excitatory postsynaptic potentials (fEPSPs)] were evoked by Schaffer collateral stimulation. Perfusion with 10 μg/ml DAAOX reduced the amplitude of the CA1 fEPSPs by 25 ± 2% (P < 0.05) 30 min after the onset of the enzyme perfusion in six slices studied. To directly determine the extent to which DAAOX perfusion affects d-serine levels in the slice, we analyzed the d-serine content of six slices treated with DAAOX. We observed a 19 ± 4% reduction in d-serine levels compared with untreated control slices. The modest effect of DAAOX on NMDA receptor responses and d-serine levels likely reflects the limited penetration of DAAOX into hippocampal slices.
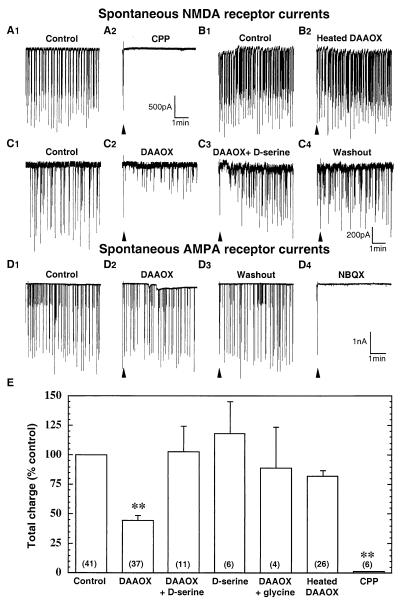
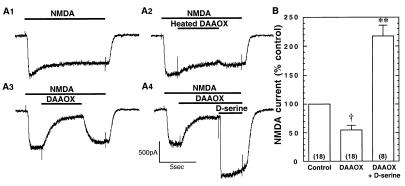
To provide enhanced access to synaptic sites during perfusion of the enzyme, primary hippocampal cell cultures were used in the remainder of the electrophysiological experiments. Whole-cell patch–clamp recordings were carried out from pyramidal-shaped neurons in 12- to 15-day-old cultures. AMPA and kainate receptor currents were blocked by inclusion of 10 μM NBQX in the recording solution. In addition, GABAA, GABAB, and glycine receptor currents were eliminated with 50 μM picrotoxin, 10 μM SCH50911, and 5 μM strychnine, respectively. We also included 5 mM QX-314 in the patch-pipette solution to block Na+ channels. Perfusion of cultured hippocampal neurons with active or heat-inactivated DAAOX fails to affect the holding current, demonstrating that the enzyme does not compromise the integrity of the cell membrane (control: 266 ± 54 pA; heat-inactivated DAAOX: 311 ± 59 pA; DAAOX: 327 ± 78 pA; n = 13, P > 0.05). We evaluated synaptically activated NMDA receptor responses by recording spontaneous NMDA receptor-mediated inward currents. Perfusion with the NMDA receptor antagonist CPP (50 μM) eliminates the currents (Fig. 4 A1 and A2), indicating that they are mediated by NMDA receptors (33, 34). Exposure for 5 min to active DAAOX (10 μg/ml) produces a significant reduction in current amplitude (Fig. 4 C1 and C2), whereas heat-inactivated DAAOX fails to affect the amplitude of NMDA receptor current events (Fig. 4 B1 and B2). When the cultures were perfused with a solution containing DAAOX plus d-serine, there was a time-dependent reversal of the inhibitory effect of DAAOX. In this case, excess d-serine (100 μM) was used to overcome the activity of the DAAOX, which degraded only ≈10% of the d-serine added to the DAAOX solution (data not shown). Glycine (100 μM) also reverses the inhibitory effect of DAAOX (Fig. 4E). DAAOX does not affect non-NMDA receptor-evoked currents recorded without NBQX and in the presence of 50 μM CPP (Fig. 4 D1– D4). The CPP-resistant currents are eliminated by 10 μM NBQX, demonstrating that they are dependent on AMPA or kainate receptors.
Figure 4.
DAAOX reduces spontaneous NMDA receptor-mediated synaptic currents in cultured hippocampal neurons. Whole-cell spontaneous excitatory postsynaptic currents (EPSCs) were recorded in pyramidal neurons of embryonic rat hippocampal in monolayer culture. Inward currents are downward. Triangles indicate artifacts resulting from valve opening for solution changes. (A1 and A2) CPP (50 μM) abolishes the synaptic currents, indicating that they are mediated by NMDA receptors. Five-minute exposure to heated DAAOX (10 μg/ml) fails to affect the synaptic currents (B1 and B2) whereas active 10 μg/ml DAAOX (C1 and C2) reduces the peak synaptic current amplitude by more than 50%. (C3 and C4) d-Serine (100 μM) plus DAAOX fully reversed the inhibitory effect of the enzyme. (D1– D4) Spontaneous AMPA receptor currents, which are blocked by 10 μM NBQX, are not affected by DAAOX application. (E) Summary of results of experiments similar to those of A–C analyzed as described in Results. Numbers of cells for each condition are shown in parentheses. Values are expressed as mean ± SEM of the control values. Statistical significance was evaluated by using ANOVA analysis followed by Scheffé posthoc comparison. (**, P < 0.0001.)
Total charge carried by NMDA receptors was quantified in 5-min epochs by power spectral analysis. The epochs were acquired 5 min after treatment with DAAOX, d-serine, or a combination of the two. As shown in Fig. 4E, active DAAOX (10 μg/ml) reduces by 55% the total charged carried by NMDA receptors, whereas heat-inactivated DAAOX has no significant effect. The inhibitory effect of DAAOX is eliminated by 100 μM d-serine, and, interestingly, 100 μM d-serine by itself fails to significantly enhance the total NMDA receptor current above basal level. This latter observation suggests that the glycine site of synaptic NMDA receptors is largely saturated under these experimental conditions.
To directly assess whether DAAOX can affect postsynaptic NMDA receptor responsiveness, NMDA receptor currents were evoked by using a multibarrel fast-perfusion system. This allowed us to investigate the time course of DAAOX action and its reversal by d-serine. Application of 100 μM CPP rapidly (τ = 186 ± 23 ms) and completely blocks the NMDA-evoked currents, demonstrating that they are dependent on NMDA receptor activation (data not shown). Coapplication of active (but not heat-inactivated) DAAOX during NMDA perfusion results in a slower (τ = 1,937 ± 292 ms) decrease in the NMDA-evoked current to a steady-state level that, on average, is 45 ± 8% less than the pre-DAAOX control value (Fig. 5 A1– A3 and B). In 10 of 18 cells, the decrement was >75% (as in the example of Fig. 5 A3– A4), whereas in the remaining 8 cells there was <10% decrement. The variability is likely due to differences in the local concentrations of d-serine among the cultured neurons. Inhibition by DAAOX recovers rapidly, presumably because local levels of coagonist can be replenished by diffusion from adjacent cells that were not affected by the local enzyme application. To evaluate the specificity of the inhibitory effect of DAAOX, we coapplied excess d-serine (100 μM), which completely reverses the inhibitory action of DAAOX and augments the current response 2-fold (Fig. 5 A4 and B). Thus, when cultured hippocampal neurons are perfused continuously, glycine sites on nonsynaptic NMDA receptors are not fully saturated (see also Fig. 6). This contrasts with the situation for synaptic NMDA receptor responses (Fig. 4) and NMDA receptor-evoked NOS activity and cGMP formation (Fig. 3 A and B). At the synapse, d-serine appears to saturate NMDA receptors, raising the possibility that nonsynaptic d-serine is washed away more easily than is the d-serine that has access to NMDA receptors during synaptic transmission. This latter pool of d-serine presumably is sequestered within the synaptic compartment or by adjacent glial cells.
Figure 5.
DAAOX inhibition and d-serine reversal of NMDA-evoked currents in cultured hippocampal neurons. Solutions were applied by using a multibarrel fast-perfusion device. Tetrodotoxin (1 μM) was present in the perfusion media. (A1) Representative current evoked by 10 μM NMDA. (A2) Heated DAAOX minimally affects the current amplitude. (A3) DAAOX (10 μg/ml) produces a 75% reduction in the current amplitude that recovers slowly upon termination of the DAAOX perfusion. (A4) d-Serine (100 μM) rapidly overcomes the DAAOX and induces a marked potentiation of the NMDA-evoked current. (B) Summary of data from 18 experiments similar to that of A. Acute application of DAAOX produced a significant decrease in the magnitude of inward current (†, P = 0.0014), whereas heat-inactivated DAAOX application did not alter significantly NMDA-evoked current (not shown). DAAOX-induced inhibition of NMDA current is reversed and the current is enhanced further by d-serine (**, P < 0.0001). Total number of recordings for each condition is indicated in parentheses. Bars indicate mean ± SEM of the maximal response expressed as a percentage of control. Statistical significance was evaluated by using ANOVA analysis followed by Scheffé posthoc comparison.
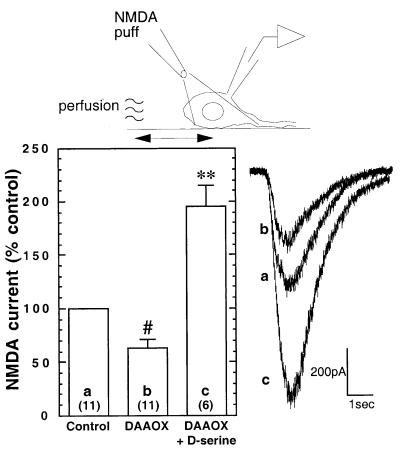
Figure 6.
DAAOX reduces NMDA receptor currents evoked with the micropuffer perfusion technique in cultured hippocampal neurons. To avoid washing away endogenous d-serine, NMDA (100 μM, 1 sec) was applied by gentle pressure ejection from a puffer pipette located ≈50 μm from the cell soma. Perfusion with DAAOX (1–10 μg/ml) attenuates the response to NMDA. The effect of DAAOX is fully reversed by the application of d-serine (100 μM), which also potentiates the NMDA-evoked current response. Recordings were in the presence of 1 μM tetrodotoxin. Values are expressed as mean ± SEM percent change in peak current amplitude with respect to control. Total number of recordings for each condition are shown in parentheses. Representative traces are shown to the right. Statistical significance was evaluated by using ANOVA analysis followed by Scheffé posthoc comparison. #, P < 0.05; **, P < 0.0001.
Because our fast perfusion system may wash away endogenous coagonist, we carried out further experiments in which NMDA was applied by using a micropuffer fluid application technique that allowed NMDA to access the cell gently with minimal bulk fluid flow. Brief pulses of NMDA (1 sec) were applied to the soma of the patched cell by pressure ejection from a micropipette positioned ≈50 μm away. As in the experiments using fast perfusion, coapplication of DAAOX (1–10 μg/ml) significantly attenuated the amplitude of NMDA-evoked currents (Fig. 6). Coapplication of DAAOX with excess d-serine (100 μM) potentiated the evoked current amplitude about 2-fold, confirming the observations with fast perfusion and indicating that the fast-perfusion system does not wash away all of the endogenous coagonist. Moreover, the results with both techniques indicate that, under the conditions of our experiments, glycine sites on extrasynaptic NMDA receptors are not fully saturated.
We confirmed by HPLC that DAAOX depletes d-serine in hippocampal cultures as it does in cerebellar slices with a 60% decline at 5 min and complete depletion at 15 min (data not shown). In culture medium exposed to hippocampal neurons for 7 and 14 days, d-serine accumulates whereas in the unconditioned medium there is no d-serine, indicating that d-serine comes from the cells and not from contaminating d-serine in the medium.
Discussion
The main finding of the present study is that DAAOX selectively attenuates NMDA receptor function monitored both biochemically and electrophysiologically, under conditions in which the enzyme selectively degrades d-serine without affecting other amino acids levels, strongly supporting a role for d-serine as an endogenous coagonist for NMDA receptors. Are synaptic levels of d-serine sufficient to physiologically occupy NMDA receptors? Total brain d-serine levels are only 40% of those of glycine, but concentrations at the receptor in situ are not known. d-Serine is up to 3-fold more effective than glycine at recombinant NMDA receptors in Xenopus oocytes (10). Extracellular d-serine levels are similar or even greater than glycine in some brain areas as measured by in vivo microdialysis (18). Moreover, d-serine is functionally 100-fold more effective than glycine at potentiating NMDA receptor-mediated spontaneous synaptic currents in hypoglossal motoneurons (14). Whether the glycine site normally is fully saturated has been unclear (35–37). However, the failure of d-serine to potentiate NMDA-evoked NOS activity and cGMP formation in the absence of DAAOX suggests that it is fully saturated under the conditions of our experiments. This conclusion is supported further by our experiments with hippocampal cell cultures. DAAOX reduced the amplitude of spontaneous NMDA receptor-mediated synaptic currents with no effect on the pattern of activity, implying a postsynaptic effect. The inhibitory effect of DAAOX was fully reversed by exogenous d-serine. However, d-serine failed to potentiate NMDA receptor synaptic responses, confirming that the NMDA receptor glycine site is largely occupied. When we examined NMDA receptor currents activated by exogenously applied NMDA (presumably carried largely by “extrasynaptic” NMDA receptors), DAAOX also was able to reduce NMDA receptor activity. This occurred whether the agonist was applied directly by using a conventional fast-perfusion technique or with the micropuffer technique that attempts to avoid washing d-serine from NMDA receptors. However, in contrast to the situation with synaptic NMDA receptors, d-serine not only reversed the action of DAAOX, but also produced a substantial increase in the amplitude of the recorded currents. We interpret this difference to indicate that extrasynaptic NMDA receptors are not fully occupied by d-serine or another glycine site agonist in those experiments.
Although our evidence is consistent with d-serine as the major endogenous ligand for the glycine site of NMDA receptors, NMDA responses were not fully blocked by the addition of DAAOX. The DAAOX-insensitive fraction varied depending on the experimental conditions, ranging from 30% to 50% of NMDA receptor activity. Endogenous glycine might occupy the remainder of the sites, and, in some parts of the brain, glycine may well be the major endogenous ligand. Evidence regarding the relative roles of these two amino acids in various brain regions derives from immunohistochemical studies in which we mapped both glycine and d-serine (7). In the adult cerebral cortex, where NMDA receptor-mediated neurotransmission is particularly prominent, neuronal levels of glycine are extremely low (7, 18). On the other hand, in the adult cerebellum, d-serine levels are low and occur only in Bergmann glia. Transmission at NMDA receptors takes place to a major extent on dendrites of granule cells. Endogenous glycine in Golgi type II neurons may activate glycine sites of the granule cell NMDA receptors (7). In the adult rat cerebellum, DAAOX fails to affect NMDA receptor activity (Fig. 3), providing additional support for the proposal that d-serine is a developmentally regulated modulator in this brain region (7).
One reason for the skepticism about a biological function in mammals for d-amino acids has been the absence of any demonstrated biosynthetic pathway. Recently, we purified (19) and cloned (38) mammalian serine racemase, which directly converts l-serine to d-serine. Serine racemase is localized to the same astrocytes as d-serine in the vicinity of NMDA receptors (38). Thus, astrocytes may play active roles in supporting synaptic transmission via the activation of NMDA receptors (39–42).
Acknowledgments
We thank Carlos D. Aizenman for continuous support during this work, Patrick Harty for helpful discussion, Adolfo Saiardi for technical assistance, Karen Wayns for assistance with the cell cultures, and Lifang Mao, who performed some initial electrophysiological studies. This work was supported by U.S. Public Health Service Grant MH-18501, the Theodore and Vada Stanley Foundation, and Research Scientist Award DA00074 (to S.H.S.); a Howard Hughes Fellowship for Physicians (to C.D.F.); and a postdoctoral fellowship from Elf Aquitaine, Inc. (to J.P.M.). H.W. is a Pew Fellow.
Abbreviations
- NMDA
N-methyl-d-aspartate
- DAAOX
d-amino acid oxidase
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline
- CPP
3-[(±)-2-carboxypiperazin-4-yl]propyl-1-phosphonate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NOS
nitric-oxide synthase
References
- 1.Hashimoto A, Oka T. Prog Neurobiol. 1997;52:325–353. doi: 10.1016/s0301-0082(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 2.Dunlop D S, Neidle A, McHale D, Dunlop D M, Lajtha A. Biochem Biophys Res Commun. 1986;141:27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto A, Nishikawa T, Oka T, Hayashi T, Takahashi K. FEBS Lett. 1993;331:4–8. doi: 10.1016/0014-5793(93)80286-4. [DOI] [PubMed] [Google Scholar]
- 4.Schell M J, Cooper O B, Snyder S H. Proc Natl Acad Sci USA. 1997;94:2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto A, Nishikawa T, Oka T, Takahashi K. J Neurochem. 1993;60:783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 6.Schell M J, Molliver M E, Snyder S H. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell M J, Brady R O, Jr, Molliver M E, Snyder S H. J Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleckner N W, Dingledine R. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 9.Wroblewski J T, Fadda E, Mazzetta J, Lazarewicz J W, Costa E. Neuropharmacology. 1989;28:447–452. doi: 10.1016/0028-3908(89)90077-4. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. J Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- 11.Paudice P, Gemignani A, Raiteri M. Eur J Neurosci. 1998;10:2934–2944. doi: 10.1046/j.1460-9568.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson J W, Ascher P. Nature (London) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 13.Sather W, Dieudonne S, MacDonald J F, Ascher P. J Physiol (London) 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger A J, Dieudonne S, Ascher P. J Neurophysiol. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- 15.Kuryatov A, Laube B, Betz H, Kuhse J. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 16.Williams K, Chao J, Kashiwagi K, Masuko T, Igarashi K. Mol Pharmacol. 1996;50:701–708. [PubMed] [Google Scholar]
- 17.Ivanovic A, Reilander H, Laube B, Kuhse J. J Biol Chem. 1998;273:19933–19937. doi: 10.1074/jbc.273.32.19933. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto A, Oka T, Nishikawa T. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- 19.Wolosker H, Sheth K N, Takahashi M, Mothet J P, Brady R O, Jr, Ferris C D, Snyder S H. Proc Natl Acad Sci USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 22.Donevan S D, Jones S M, Rogawski M A. Mol Pharmacol. 1992;41:727–735. [PubMed] [Google Scholar]
- 23.D'Aniello A, Vetere A, Petrucelli L. Comp Biochem Physiol B. 1993;105:731–734. doi: 10.1016/0305-0491(93)90113-j. [DOI] [PubMed] [Google Scholar]
- 24.Denu J M, Fitzpatrick P F. J Biol Chem. 1994;269:15054–15059. [PubMed] [Google Scholar]
- 25.Southam E, East S J, Garthwaite J. J Neurochem. 1991;56:2072–2081. doi: 10.1111/j.1471-4159.1991.tb03468.x. [DOI] [PubMed] [Google Scholar]
- 26.Wei J W, Chang C M, Chang L W. Int J Biochem. 1993;25:1579–1585. doi: 10.1016/0020-711x(93)90515-g. [DOI] [PubMed] [Google Scholar]
- 27.Goel R, Mishra O P, Razdan B, Delivoria-Papadopoulos M. Neurosci Lett. 1993;151:219–223. doi: 10.1016/0304-3940(93)90024-f. [DOI] [PubMed] [Google Scholar]
- 28.Honore T, Davies S N, Drejer J, Fletcher E J, Jacobsen P, Lodge D, Nielsen F E. Science. 1988;241:701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Mifflin S W. J Physiol (London) 1998;511:733–745. doi: 10.1111/j.1469-7793.1998.733bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleakman D, Lodge D. Neuropharmacology. 1998;37:1187–1204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 31.Baron B M, Harrison B L, Miller F P, McDonald I A, Salituro F G, Schmidt C J, Sorensen S M, White H S, Palfreyman M G. Mol Pharmacol. 1990;38:554–561. [PubMed] [Google Scholar]
- 32.Foster A C, Kemp J A, Leeson P D, Grimwood S, Donald A E, Marshall G R, Priestey T, Smith J D, Carling R W. Mol Pharmacol. 1992;41:914–922. [PubMed] [Google Scholar]
- 33.Davies J, Evans R H, Herrling P L, Jones A W, Oliverman H J, Pook P, Watkins J C. Brain Res. 1986;382:169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann J, Schneider J, McPherson S, Murphy D E, Bernard P, Tsai C, Bennett D A, Pastor G, Steel D J, Boehm C, et al. J Pharmacol Exp Ther. 1987;240:737–746. [PubMed] [Google Scholar]
- 35.Tang C M, Margulis M, Shi Q Y, Fielding A. Neuron. 1994;13:1385–1393. doi: 10.1016/0896-6273(94)90423-5. [DOI] [PubMed] [Google Scholar]
- 36.Mainen Z F, Malinow R, Svoboda K. Nature (London) 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- 37.Danysz W, Parsons A C G. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- 38.Wolosker H, Blackshaw S, Snyder S H. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedergaard M. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 40.Pfrieger F W, Barres B A. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 41.Araque A, Sanzgiri R P, Parpura V, Haydon P G. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bacci A, Verderio C, Pravettoni E, Matteoli M. Philos Trans R Soc London B. 1999;354:403–409. doi: 10.1098/rstb.1999.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]