Abstract
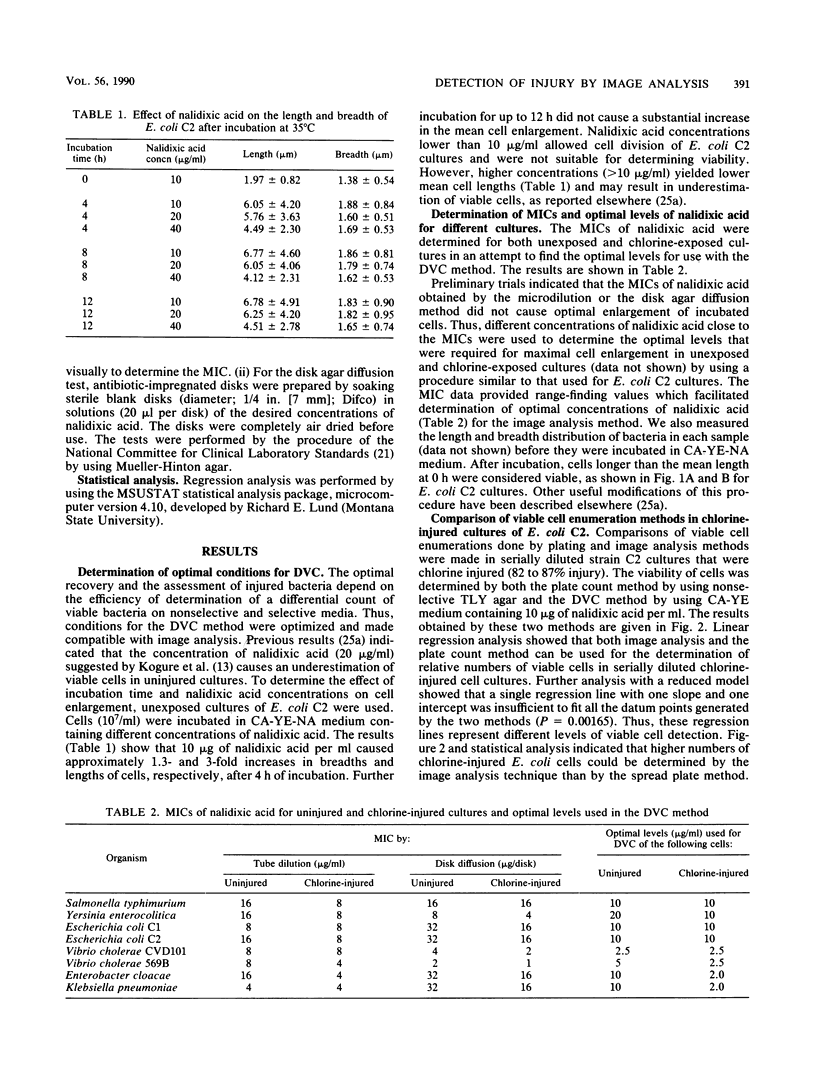
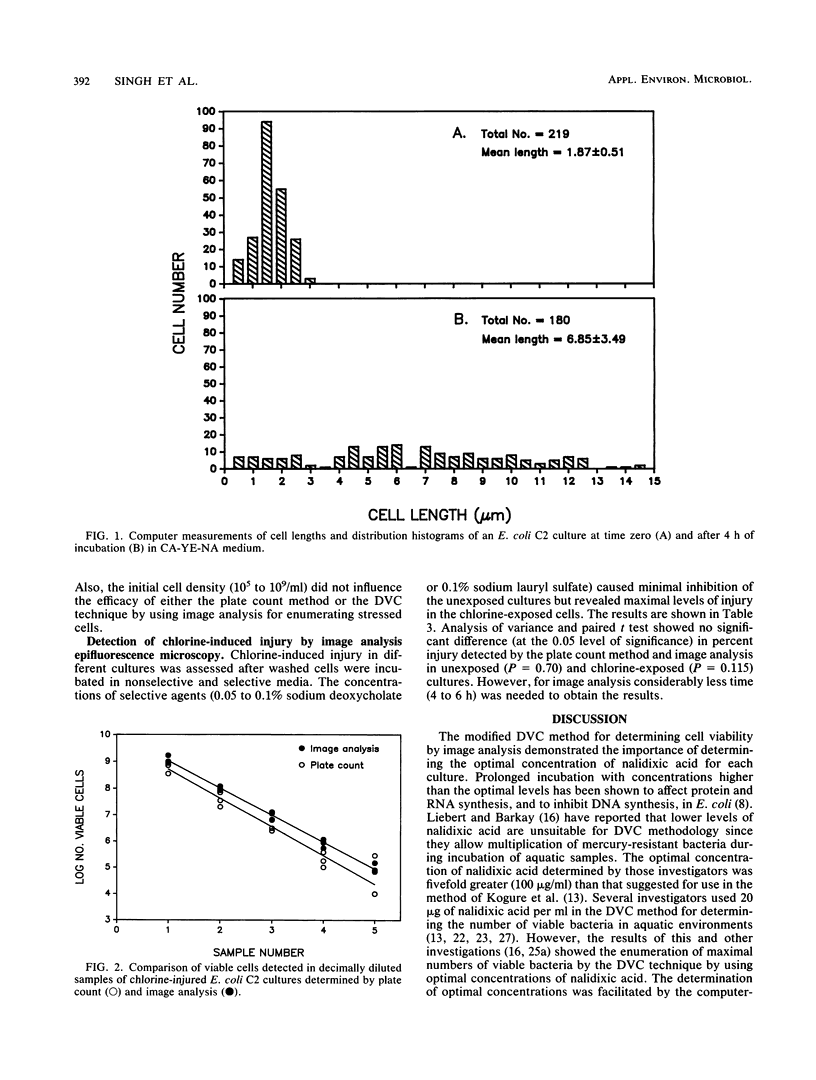
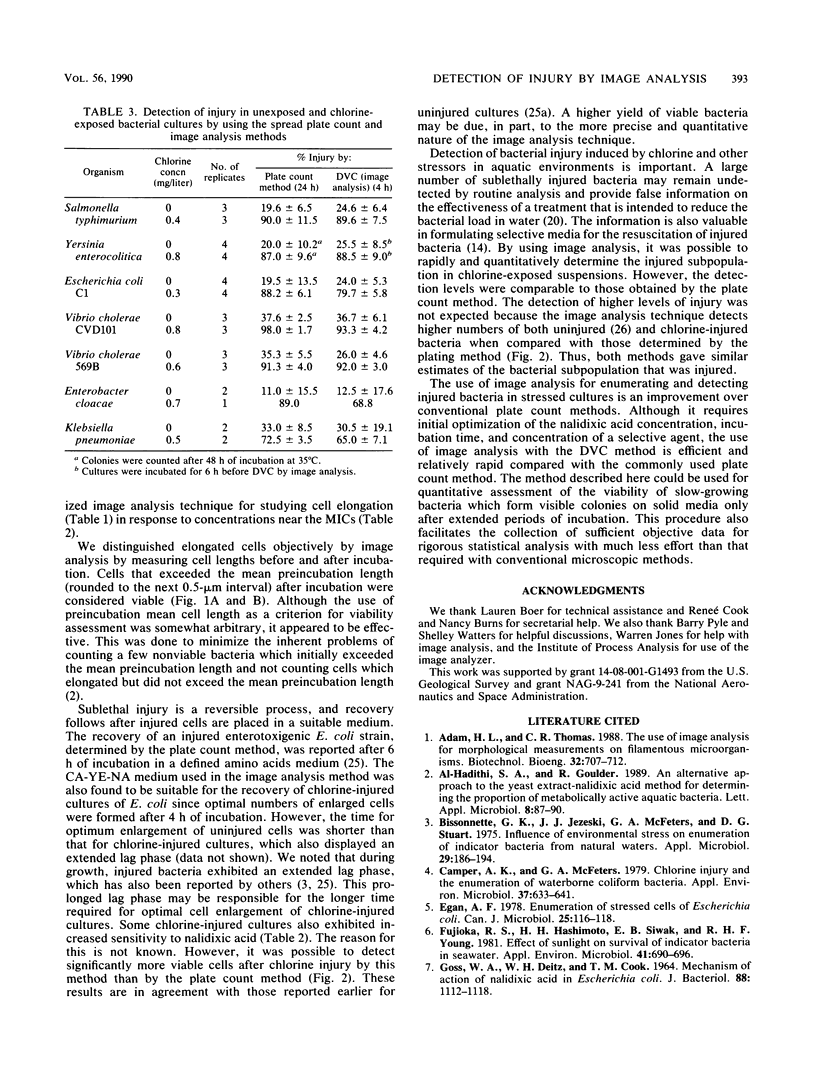
A modified direct viable count method to detect living bacteria was used with image analysis for the rapid enumeration of chlorine-injured cells in an Escherichia coli culture. The method was also used for determining chlorine-induced injury in coliform isolates and enteric pathogenic bacteria. Cultures were incubated in phosphate-buffered saline, containing 0.3% Casamino Acids (Difco Laboratories, Detroit, Mich.), 0.03% yeast extract, and optimal concentrations of nalidixic acid. Samples were withdrawn before and after incubation and stained with acridine orange, and cell lengths and breadths were measured by computerized image analysis. After incubation, cells which exceeded the mean preincubation length (viable cells) were enumerated and the results were compared with those obtained by the plate count method. Injury in the chlorine-exposed cell population was determined from the difference in viable count obtained with a nonselective Casamino Acids-yeast extract-nalidixic acid medium and a selective Casamino Acids-yeast extract-nalidixic acid medium containing sodium deoxycholate or sodium lauryl sulfate. The levels of injury determined by the direct viable count technique by using image analysis were comparable to those determined by the plate count method. The results showed that image analysis, under optimal conditions, enumerated significantly higher numbers of stressed E. coli than the plate count method did and detected injury in various cultures in 4 to 6 h.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper A. K., McFeters G. A. Chlorine injury and the enumeration of waterborne coliform bacteria. Appl Environ Microbiol. 1979 Mar;37(3):633–641. doi: 10.1128/aem.37.3.633-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan A. F. Enumeration of stressed cells of Escherichia coli. Can J Microbiol. 1979 Jan;25(1):116–118. doi: 10.1139/m79-018. [DOI] [PubMed] [Google Scholar]
- Fujioka R. S., Hashimoto H. H., Siwak E. B., Young R. H. Effect of sunlight on survival of indicator bacteria in seawater. Appl Environ Microbiol. 1981 Mar;41(3):690–696. doi: 10.1128/aem.41.3.690-696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol. 1968 Feb;95(2):520–530. doi: 10.1128/jb.95.2.520-530.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- LeChevallier M. W., Cameron S. C., McFeters G. A. New medium for improved recovery of coliform bacteria from drinking water. Appl Environ Microbiol. 1983 Feb;45(2):484–492. doi: 10.1128/aem.45.2.484-492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Cameron S. C., LeChevallier M. W. Influence of diluents, media, and membrane filters on detection fo injured waterborne coliform bacteria. Appl Environ Microbiol. 1982 Jan;43(1):97–103. doi: 10.1128/aem.43.1.97-103.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Camper A. K. Enumeration of indicator bacteria exposed to chlorine. Adv Appl Microbiol. 1983;29:177–193. doi: 10.1016/s0065-2164(08)70357-5. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Kippin J. S., LeChevallier M. W. Injured coliforms in drinking water. Appl Environ Microbiol. 1986 Jan;51(1):1–5. doi: 10.1128/aem.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., LeChevallier M. W., Singh A., Kippin J. S. Health significance and occurrence of injured bacteria in drinking water. Water Sci Technol. 1986;18(10):227–231. [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Schiff L. J., Morrison S. M., Mayeux J. V. Synergistic false-positive coliform reaction on M-Endo MF medium. Appl Microbiol. 1970 Nov;20(5):778–781. doi: 10.1128/am.20.5.778-781.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., McFeters G. A. Recovery, growth, and production of heat-stable enterotoxin by Escherichia coli after copper-induced injury. Appl Environ Microbiol. 1986 Apr;51(4):738–742. doi: 10.1128/aem.51.4.738-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Pyle B. H., McFeters G. A. Rapid enumeration of viable bacteria by image analysis. J Microbiol Methods. 1989;10:91–101. doi: 10.1016/0167-7012(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Singh A., Yeager R., McFeters G. A. Assessment of in vivo revival, growth, and pathogenicity of Escherichia coli strains after copper- and chlorine-induced injury. Appl Environ Microbiol. 1986 Oct;52(4):832–837. doi: 10.1128/aem.52.4.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


