Abstract
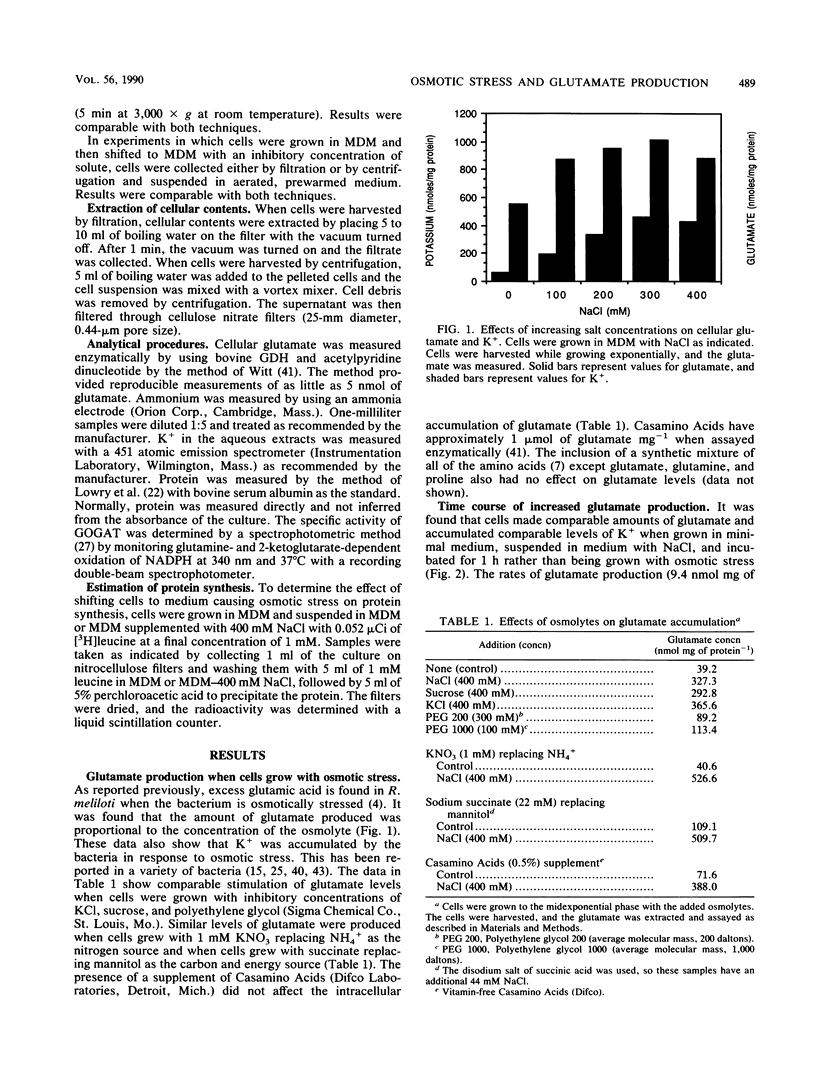
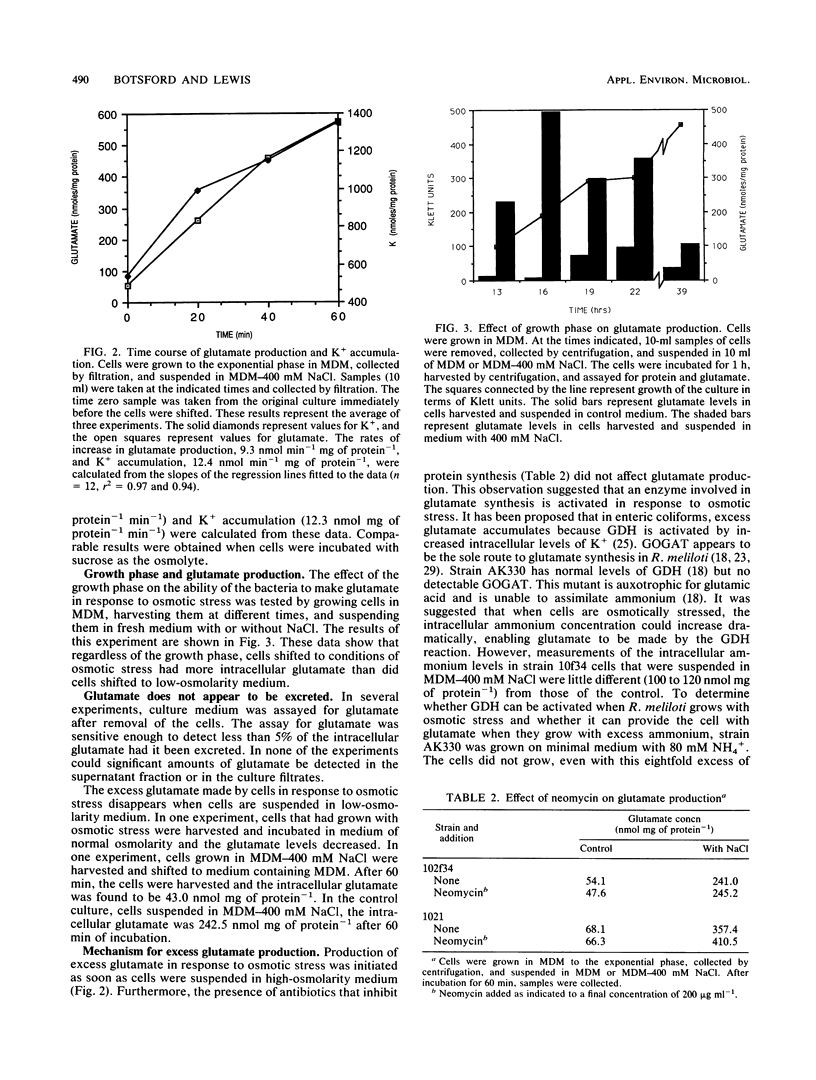
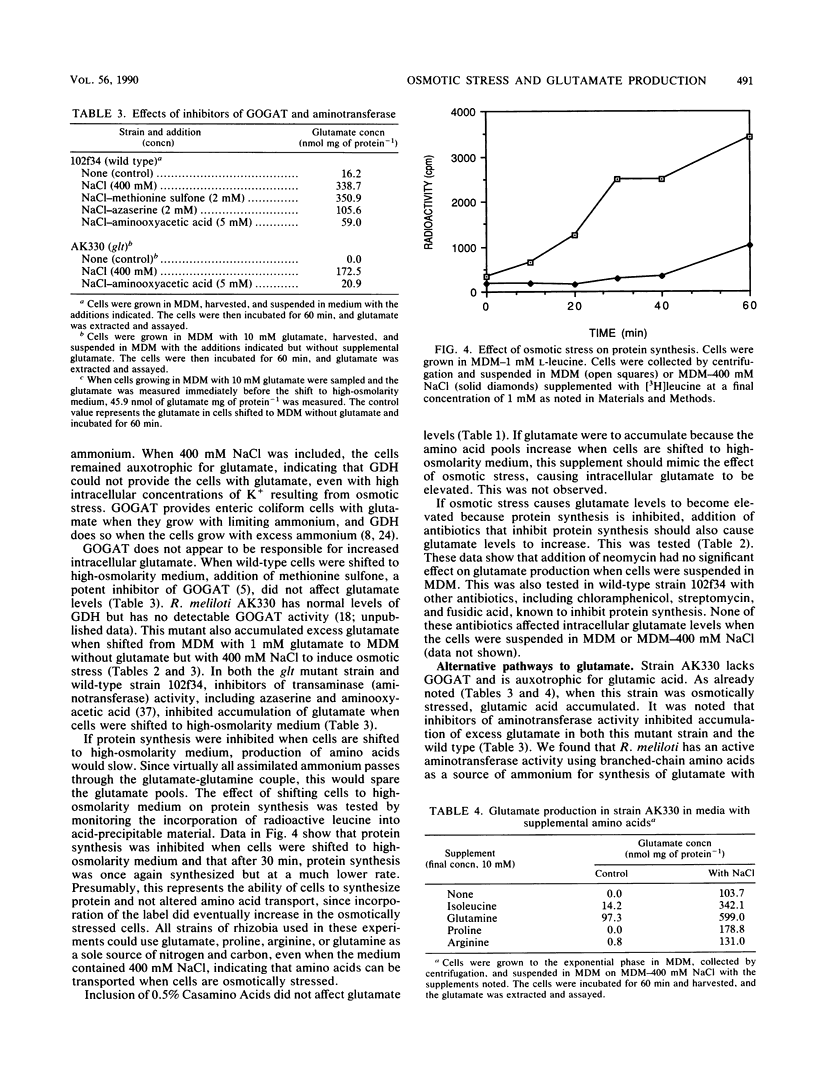
Rhizobium meliloti, like many other bacteria, accumulates high levels of glutamic acid when osmotically stressed. The effect was found to be proportional to the osmolarity of the growth medium. NaCl, KCI, sucrose, and polyethylene glycol elicited this response. The intracellular levels of glutamate and K+ began to increase immediately when cells were shifted to high-osmolarity medium. Antibiotics that inhibit protein synthesis did not affect this increase in glutamate production. Cells growing in conventional media at any stage in the growth cycle could be suspended in medium causing osmotic stress and excess glutamate accumulated. The excess glutamate did not appear to be excreted, and the intracellular level eventually returned to normal when osmotically stressed cells were suspended in low-osmolarity medium. A glt mutant lacking glutamate synthase and auxotrophic for glutamate accumulated excess glutamate in response to osmotic stress. Addition of isoleucine, glutamine, proline, or arginine stimulated glutamate accumulation to wild-type levels when the mutant cells were suspended in minimal medium with NaCl to cause osmotic stress. In both wild-type and mutant cells, inhibitors of transaminase activity, including azaserine and aminooxyacetate, reduced glutamate levels. The results suggest that the excess glutamate made in response to osmotic stress is derived from degradation of amino acids and transamination of 2-ketoglutarate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boos W., Ehmann U., Bremer E., Middendorf A., Postma P. Trehalase of Escherichia coli. Mapping and cloning of its structural gene and identification of the enzyme as a periplasmic protein induced under high osmolarity growth conditions. J Biol Chem. 1987 Sep 25;262(27):13212–13218. [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendinger S. M., Patil L. G., Brenchley J. E. Salmonella typhimurium mutants with altered glutamate dehydrogenase and glutamate synthase activities. J Bacteriol. 1980 Jan;141(1):190–198. doi: 10.1128/jb.141.1.190-198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever H. M., Styrvold O. B., Kaasen I., Strøm A. R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. K regulates bacteroid-associated functions of Bradyrhizobium. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4650–4654. doi: 10.1073/pnas.84.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. S., Tsai V. Y., Lichens G. M., Noma A. T. Accumulation of Amino Acids in Rhizobium sp. Strain WR1001 in Response to Sodium Chloride Salinity. Appl Environ Microbiol. 1982 Jul;44(1):135–140. doi: 10.1128/aem.44.1.135-140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Leirmo S., Harrison C., Cayley D. S., Burgess R. R., Record M. T., Jr Replacement of potassium chloride by potassium glutamate dramatically enhances protein-DNA interactions in vitro. Biochemistry. 1987 Apr 21;26(8):2095–2101. doi: 10.1021/bi00382a006. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Osburne M. S., Signer E. R. Ammonium assimilation in Rhizobium meliloti. J Bacteriol. 1980 Sep;143(3):1234–1240. doi: 10.1128/jb.143.3.1234-1240.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocard J. A., Bernard T., Smith L. T., Le Rudulier D. Characterization of three choline transport activities in Rhizobium meliloti: modulation by choline and osmotic stress. J Bacteriol. 1989 Jan;171(1):531–537. doi: 10.1128/jb.171.1.531-537.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Keizer H. G., Koolwijk P. Transport of trehalose in Salmonella typhimurium. J Bacteriol. 1986 Dec;168(3):1107–1111. doi: 10.1128/jb.168.3.1107-1111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey B., Cayley D. S., Mossing M. C., Kolka C., Anderson C. F., Farrar T. C., Record M. T., Jr Variability of the intracellular ionic environment of Escherichia coli. Differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J Biol Chem. 1987 May 25;262(15):7157–7164. [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Smith G. M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989 Sep;171(9):4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp S. S., Vance C. P. Asparagine Biosynthesis in Alfalfa (Medicago sativa L.) Root Nodules. Plant Physiol. 1986 Oct;82(2):390–395. doi: 10.1104/pp.82.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrvold O. B., Falkenberg P., Landfald B., Eshoo M. W., Bjørnsen T., Strøm A. R. Selection, mapping, and characterization of osmoregulatory mutants of Escherichia coli blocked in the choline-glycine betaine pathway. J Bacteriol. 1986 Mar;165(3):856–863. doi: 10.1128/jb.165.3.856-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


