Abstract
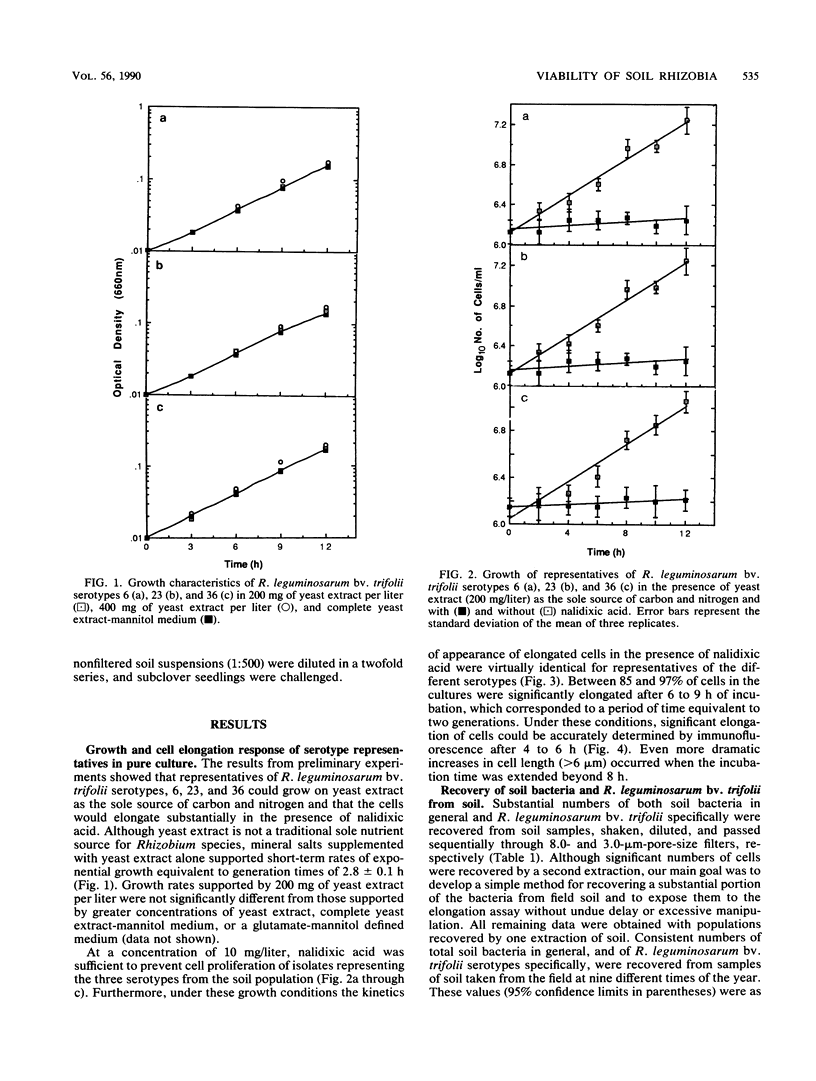
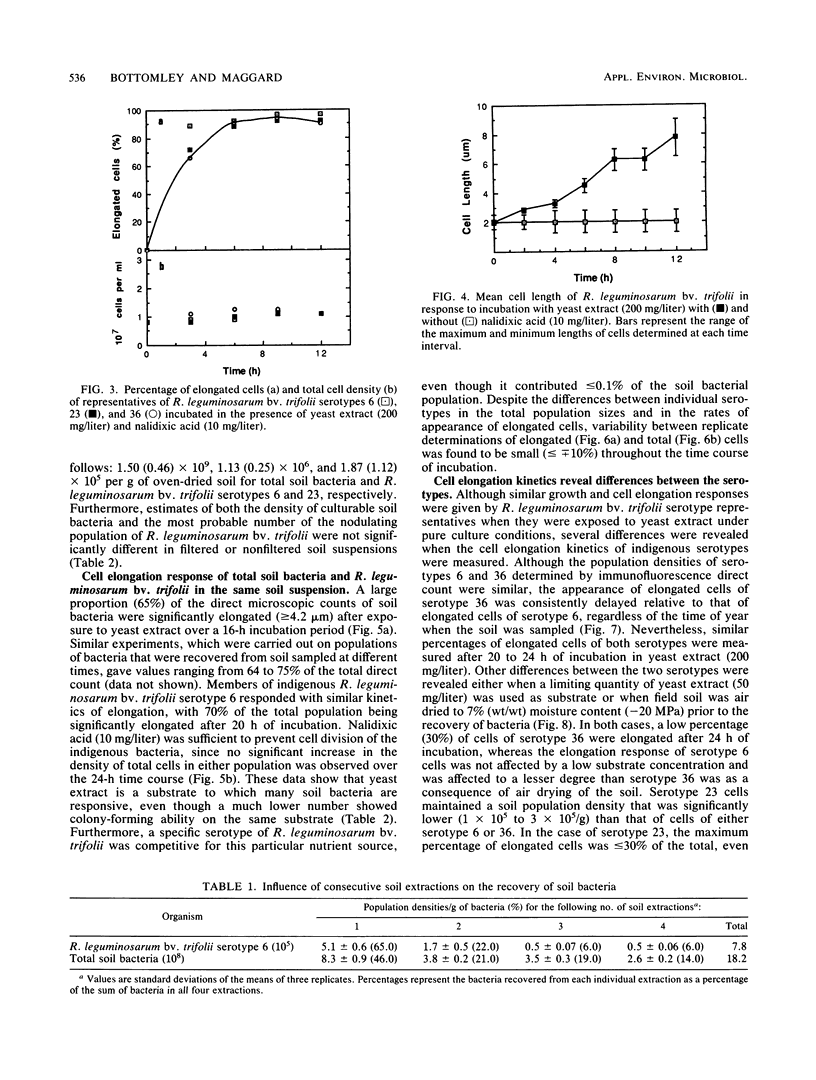
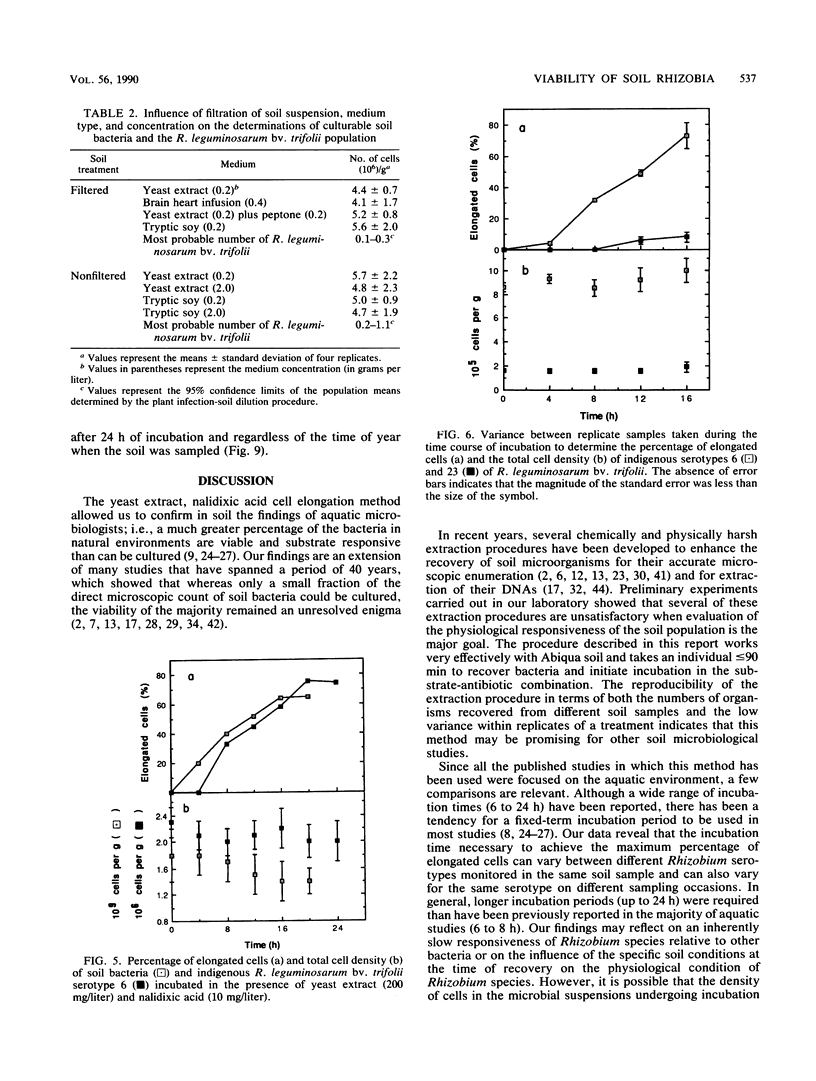
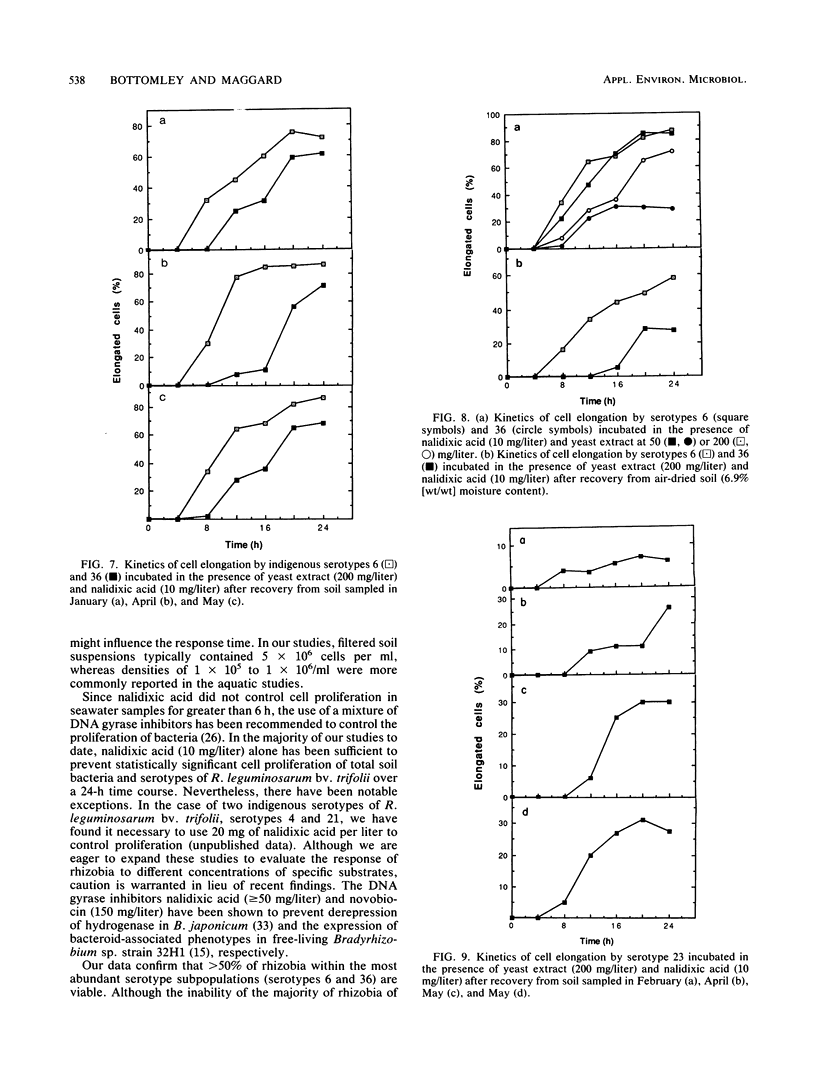
Concern has been raised about the percentage of viable cells within soil rhizobia populations measured by the immunofluorescence direct count method. The purpose of this study was to evaluate a direct viable count technique which is based on the fact that viable bacteria in natural populations undergo cell elongation when they are exposed to a combination of substrate and the inhibitor of DNA gyrase, nalidixic acid. A soil extraction procedure was developed to recover a high proportion of soil bacteria (ca. 10(9)/g of soil) in suspensions with an optical clarity suitable for accurate microscopic enumeration. After incubation for 16 to 20 h at 27 degrees C in the presence of yeast extract (200 mg/liter) and nalidixic acid (10 mg/liter), between 65 and 74% of the bacteria in soil suspension became significantly elongated (greater than or equal to 4.2 microns). In contrast, less than or equal to 0.5% of the same population could be cultured, regardless of the medium composition, nutrient concentration, or incubation conditions. The direct viable count method was combined with immunofluorescence to compare the percent viability and kinetics of appearance of elongated cells within serotypes of a soil population of Rhizobium leguminosarum bv. trifolii. Although the majority of these organisms were viable, as observed by immunofluorescence, we obtained evidence that subpopulations within the soil rhizobia community were in different states of competence to respond to substrate. A consistently low percentage (less than or equal to 30%) of the population of serotype 23 was elongated even after 24 h of incubation and regardless of when the soil was sampled.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendras A. S., Bottomley P. J. Influence of Lime and Phosphate on Nodulation of Soil-Grown Trifolium subterraneum L. by Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2090–2097. doi: 10.1128/aem.53.9.2090-2097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken L. R. Separation and purification of bacteria from soil. Appl Environ Microbiol. 1985 Jun;49(6):1482–1487. doi: 10.1128/aem.49.6.1482-1487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone T. L., Balkwill D. L. Improved flotation technique for microscopy of in situ soil and sediment microorganisms. Appl Environ Microbiol. 1986 Mar;51(3):462–468. doi: 10.1128/aem.51.3.462-468.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. J., Dughri M. H. Population Size and Distribution of Rhizobium leguminosarum bv. trifolii in Relation to Total Soil Bacteria and Soil Depth. Appl Environ Microbiol. 1989 Apr;55(4):959–964. doi: 10.1128/aem.55.4.959-964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton P. R., Tamplin M. L., Huq A., Colwell R. R. Enumeration of Vibrio cholerae O1 in Bangladesh waters by fluorescent-antibody direct viable count. Appl Environ Microbiol. 1987 Dec;53(12):2862–2865. doi: 10.1128/aem.53.12.2862-2865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Autecology in Rhizospheres and Nodulating Behavior of Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1014–1019. doi: 10.1128/aem.52.5.1014-1019.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson J. K., Bezdicek D. F., Brockman F. J., Li S. W. Enumeration of Tn5 mutant bacteria in soil by using a most- probable-number-DNA hybridization procedure and antibiotic resistance. Appl Environ Microbiol. 1988 Feb;54(2):446–453. doi: 10.1128/aem.54.2.446-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Role of DNA Superhelicity in Regulation of Bacteroid-Associated Functions of Bradyrhizobium sp. Strain 32H1. Appl Environ Microbiol. 1989 Jun;55(6):1420–1425. doi: 10.1128/aem.55.6.1420-1425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. D., Ward L. J., Slade E. A. Expression by Soil Bacteria of Nodulation Genes from Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol. 1989 Jun;55(6):1426–1434. doi: 10.1128/aem.55.6.1426-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley M. T., Bohlool B. B. Release of Rhizobium spp. from Tropical Soils and Recovery for Immunofluorescence Enumeration. Appl Environ Microbiol. 1981 Aug;42(2):241–248. doi: 10.1128/aem.42.2.241-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N., Colwell R. R. Correlation of direct viable counts with heterotrophic activity for marine bacteria. Appl Environ Microbiol. 1987 Oct;53(10):2332–2337. doi: 10.1128/aem.53.10.2332-2337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. Distribution of viable marine bacteria in neritic seawater around Japan. Can J Microbiol. 1980 Mar;26(3):318–323. doi: 10.1139/m80-052. [DOI] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P. D., Maier R. J. Inhibition of hydrogenase synthesis by DNA gyrase inhibitors in Bradyrhizobium japonicum. J Bacteriol. 1987 Jun;169(6):2708–2712. doi: 10.1128/jb.169.6.2708-2712.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINNER F. A., JONES P. C., MOLLISON J. E. A comparison of a direct- and a plate counting technique for the quantitative estimation of soil micro-organisms. J Gen Microbiol. 1952 May;6(3-4):261–271. doi: 10.1099/00221287-6-3-4-261. [DOI] [PubMed] [Google Scholar]


