Abstract
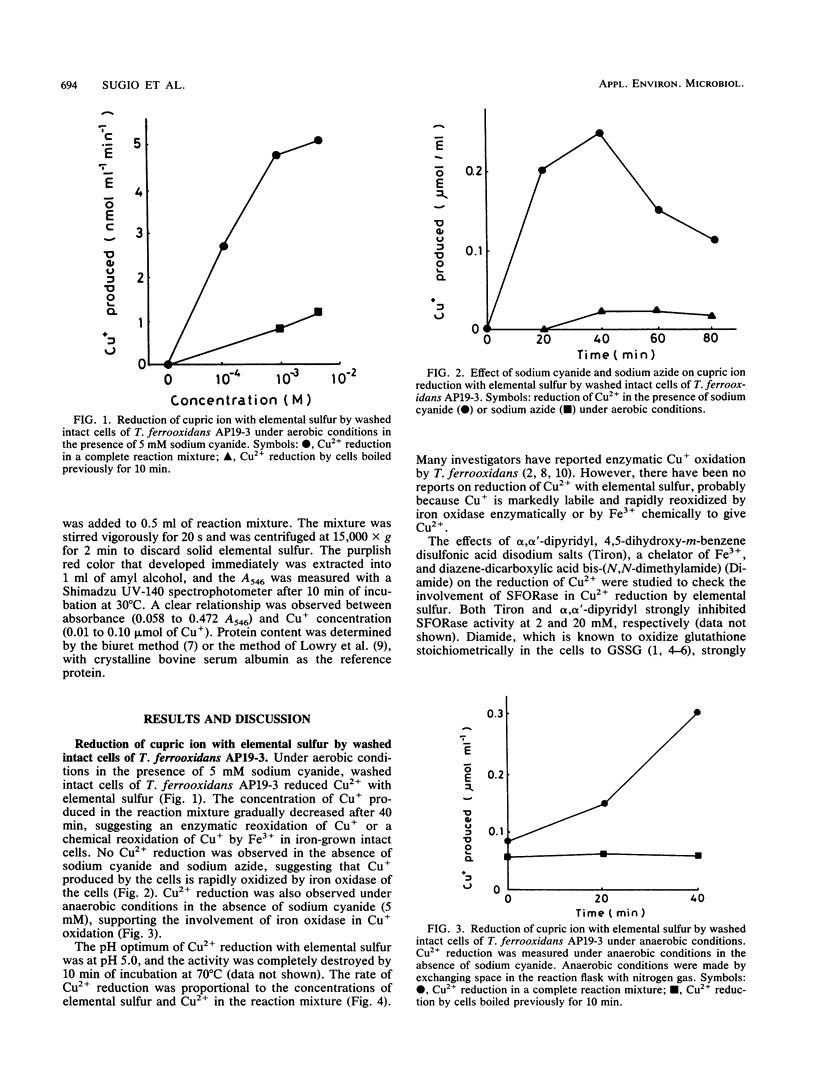
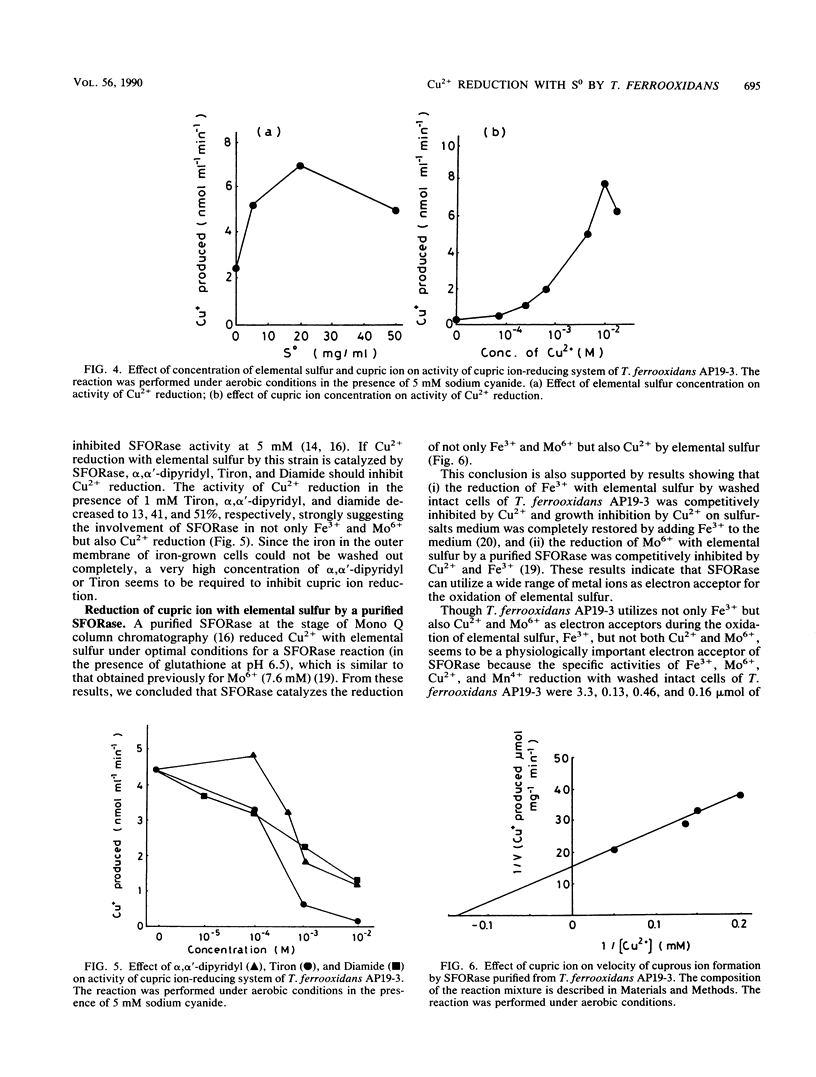
In anaerobic or aerobic conditions in the presence of 5 mM sodium cyanide, an inhibitor of iron oxidase, cupric ion (Cu2+) was reduced enzymatically with elemental sulfur (S0) by washed intact cells of Thiobacillus ferrooxidans AP19-3 to give cuprous ion (Cu+). The rate of Cu2+ reduction was proportional to the concentrations of S0 and Cu2+ added to the reaction mixture. The pH optimum for the cupric ion-reducing system was 5.0, and the activity was completely destroyed by 10-min incubation of cells at 70°C. The activity of Cu2+ reduction with S0 by this strain was strongly inhibited by inhibitors of hydrogen sulfide: ferric ion oxidoreductase (SFORase), such as α,α′-dipyridyl, 4,5-dihydroxy-m-benzene disulfonic acid disodium salts, and diazine dicarboxylic acid bis-(N, N-dimethylamide). A SFORase purified from this strain, which catalyzes oxidation of both hydrogen sulfide and S0 with Fe3+ or Mo6+ as an electron acceptor in the presence of glutathione, catalyzed a reduction of Cu2+ by S0, and the Michaelis constant of SFORase for Cu2+ was 7.2 mM, indicating that a SFORase catalyzes the reduction of not only Fe3+ and Mo6+ but also Cu2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982 Nov 30;683(2):89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S. Lest I forget thee, glutathione. Nature. 1969 Oct 11;224(5215):117–120. doi: 10.1038/224117a0. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Kosower E. M., Huang P. C. Decreased glutathione content of human erythrocytes produced by methyl phenylazoformate. Biochem Biophys Res Commun. 1965 Aug 16;20(4):469–474. doi: 10.1016/0006-291x(65)90602-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis A. J., Miller J. D. Stannous and cuprous ion oxidation by Thiobacillus ferrooxidans. Can J Microbiol. 1977 Mar;23(3):319–324. doi: 10.1139/m77-047. [DOI] [PubMed] [Google Scholar]
- Nielsen A. M., Beck J. V. Chalcocite Oxidation and Coupled Carbon Dioxide Fixation by Thiobacillus ferrooxidans. Science. 1972 Mar 10;175(4026):1124–1126. doi: 10.1126/science.175.4026.1124. [DOI] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Munakata O., Tano T., Imai K. Role of a Ferric Ion-Reducing System in Sulfur Oxidation of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1985 Jun;49(6):1401–1406. doi: 10.1128/aem.49.6.1401-1406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Tano T., Imai K. Role of Ferrous Ions in Synthetic Cobaltous Sulfide Leaching of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1984 Sep;48(3):461–467. doi: 10.1128/aem.48.3.461-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Katagiri T., Moriyama M., Zhèn Y. L., Inagaki K., Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ions as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988 Jan;54(1):153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Mizunashi W., Inagaki K., Tano T. Purification and some properties of sulfur:ferric ion oxidoreductase from Thiobacillus ferrooxidans. J Bacteriol. 1987 Nov;169(11):4916–4922. doi: 10.1128/jb.169.11.4916-4922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Tsujita Y., Katagiri T., Inagaki K., Tano T. Reduction of Mo6+ with elemental sulfur by Thiobacillus ferrooxidans. J Bacteriol. 1988 Dec;170(12):5956–5959. doi: 10.1128/jb.170.12.5956-5959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio Tsuyoshi, Wada Kimihito, Mori Manami, Inagaki Kenji, Tano Tatsuo. Synthesis of an Iron-Oxidizing System during Growth of Thiobacillus ferrooxidans on Sulfur-Basal Salts Medium. Appl Environ Microbiol. 1988 Jan;54(1):150–152. doi: 10.1128/aem.54.1.150-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


