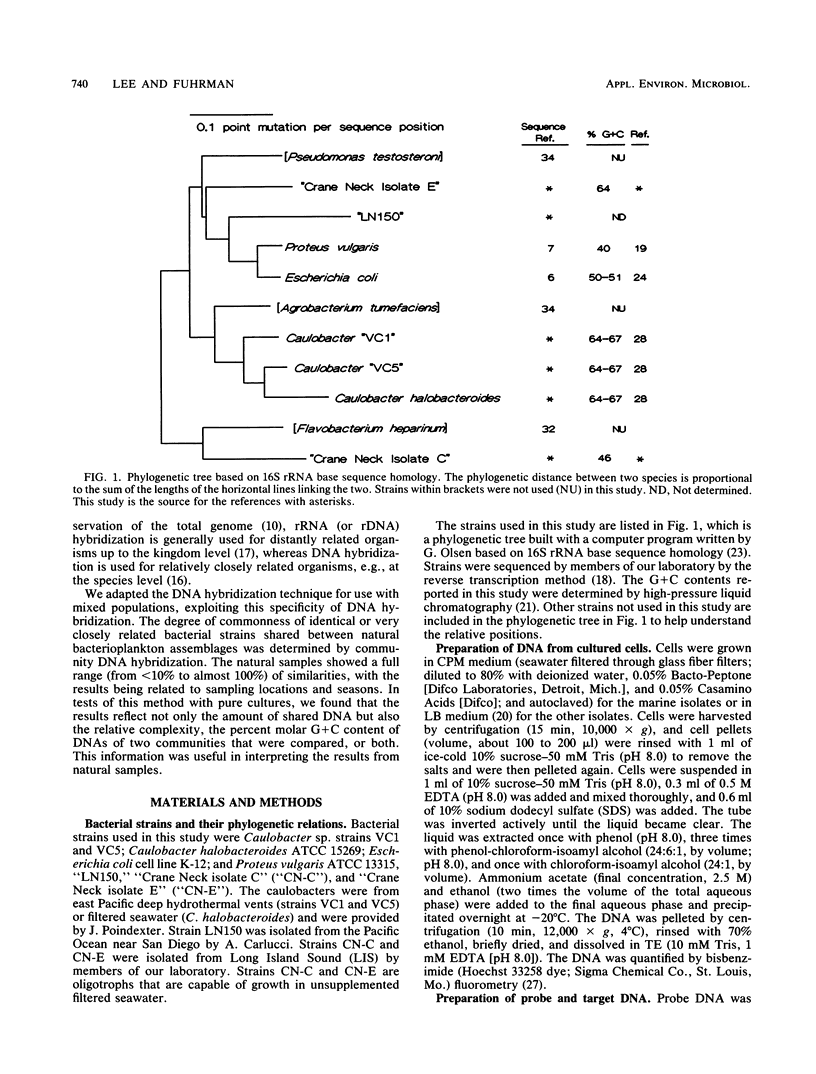
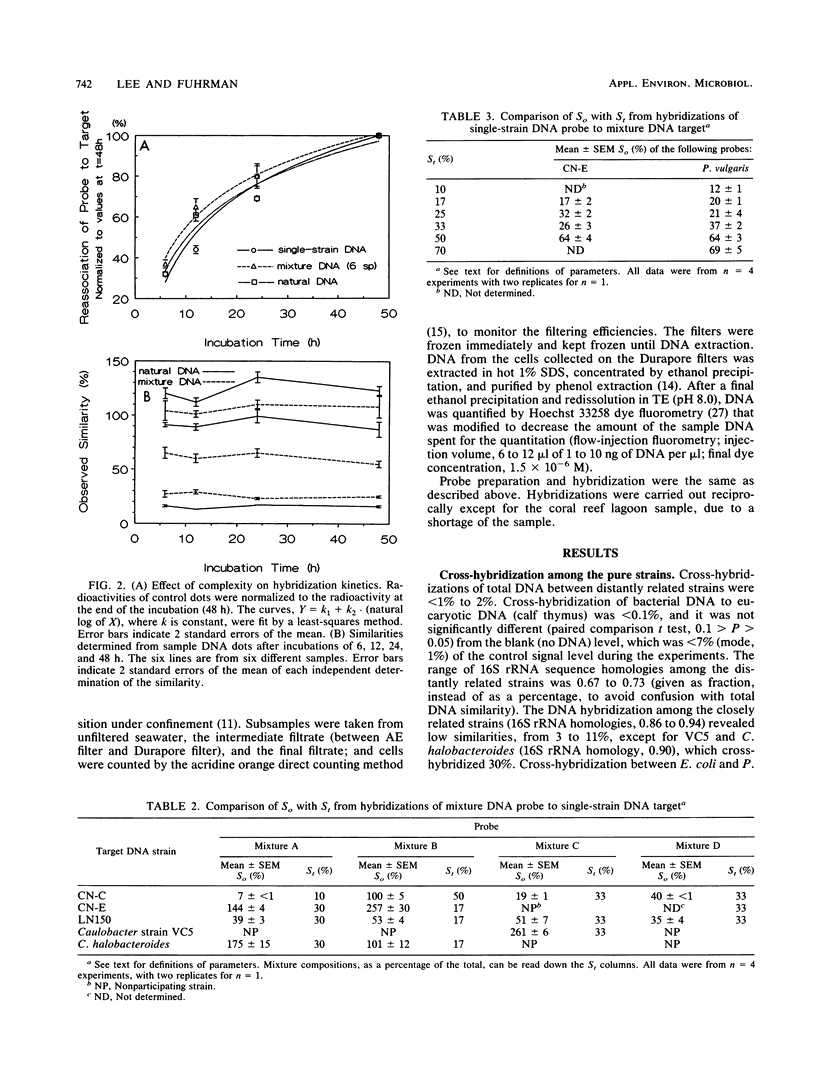
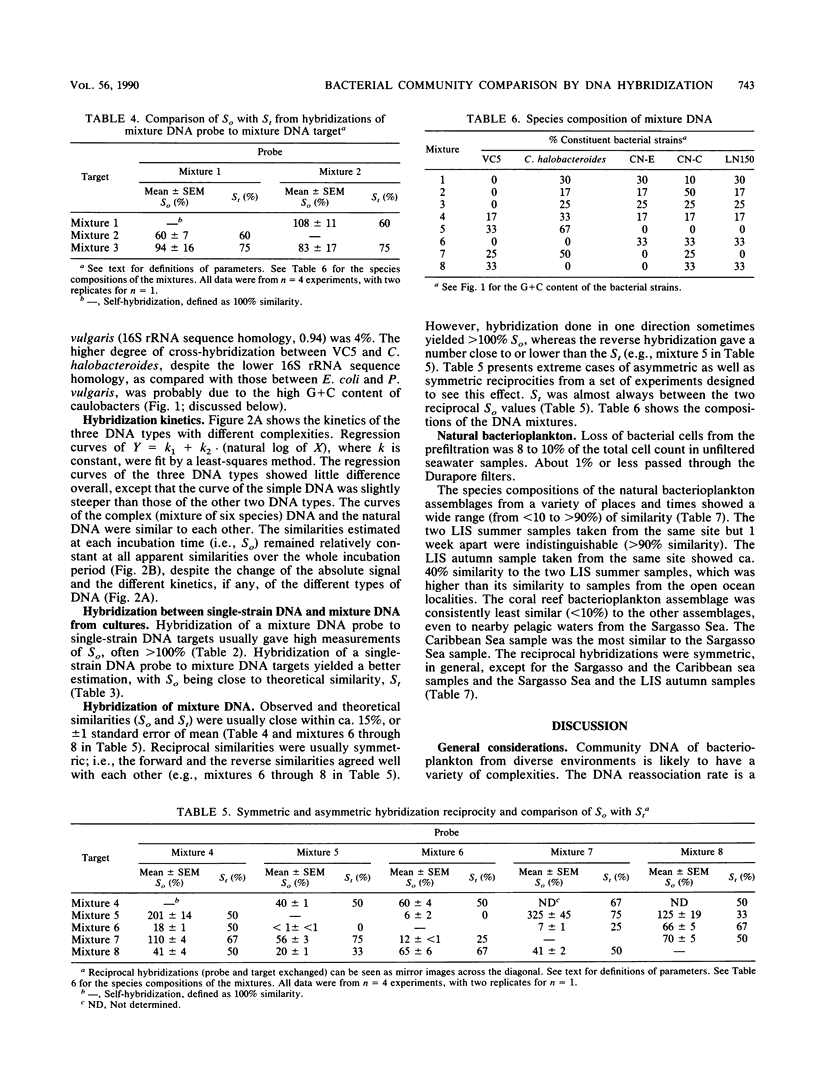
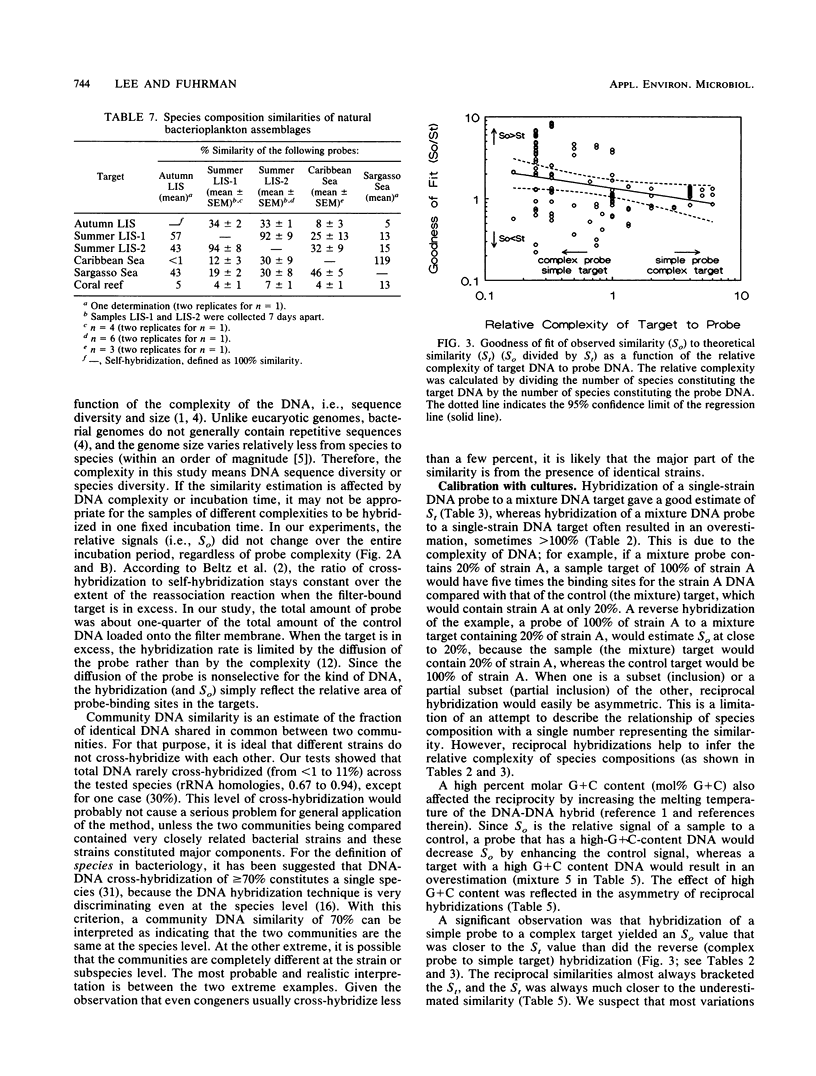
Abstract
Little is known about the species composition and variability of natural bacterial communities, mostly because conventional identification requires pure cultures, but less than 1% of active natural bacteria are cultivable. This problem was circumvented by comparing species compositions via hybridization of total DNA of natural bacterioplankton communities for the estimation of the fraction of DNA in common between two samples (similarity). DNA probes that were labeled with 35S by nick translation were hybridized to filter-bound DNA in a reciprocal fashion; similarities (in percent) were calculated by normalizing the values to self-hybridizations. In tests with DNA mixtures of pure cultures, the experimentally observed similarities agreed with expectations. However, reciprocal similarities (probe and target reversed) were often asymmetric, unlike those of DNA from single strains. This was due to the relative complexity and G + C content of DNA, which provided a means to interpret the asymmetry that was occasionally observed in natural samples. Natural bacteria were collected by filtration from Long Island Sound (LIS), N.Y., the Caribbean and Sargasso seas, and a coral reef lagoon near Bermuda. The samples showed similarities of less than 10 to 95%. The LIS and Sargasso and Caribbean sea samples were 20 to 50% similar to each other. The coral reef sample was less than 10% similar to the others, indicating its unique composition. Seasonality was also observed; an LIS sample obtained in the autumn was 40% similar to two LIS samples obtained in the summer; these latter two samples were 95% similar. We concluded that total DNA hybridization is a rapid, simple, and unbiased method for investigating the variation of bacterioplankton species composition over time and space, avoiding the need of culturing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ebel J. P., Ehresmann C. The sequence of the ribosomal 16S RNA from Proteus vulgaris. Sequence comparison with E. coli 16S RNA and its use in secondary model building. Nucleic Acids Res. 1981 May 25;9(10):2325–2333. doi: 10.1093/nar/9.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Birfelder E. J., Sanders J. P., Borst P. DNA-DNA hybridization on nitrocellulose filters. 1. General considerations and non-ideal kinetics. Eur J Biochem. 1974 Sep 16;47(3):535–543. doi: 10.1111/j.1432-1033.1974.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Comeau D. E., Hagström A., Chan A. M. Extraction from natural planktonic microorganisms of DNA suitable for molecular biological studies. Appl Environ Microbiol. 1988 Jun;54(6):1426–1429. doi: 10.1128/aem.54.6.1426-1429.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. J., Field K. G., Olsen G. J., Pace N. R. Reverse transcriptase sequencing of ribosomal RNA for phylogenetic analysis. Methods Enzymol. 1988;167:138–144. doi: 10.1016/0076-6879(88)67015-7. [DOI] [PubMed] [Google Scholar]
- Mischke C. F., Wickstrom E. Deoxynucleoside composition of DNAs and modified nucleoside composition of tRNAs determined at nanomole sensitivity by reversed-phase liquid chromatography. Anal Biochem. 1980 Jun;105(1):181–187. doi: 10.1016/0003-2697(80)90443-1. [DOI] [PubMed] [Google Scholar]
- Olsen G. J. Phylogenetic analysis using ribosomal RNA. Methods Enzymol. 1988;164:793–812. doi: 10.1016/s0076-6879(88)64084-5. [DOI] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Lynn S. P., Gardner J. F. Use of randomly cloned DNA fragments for identification of Bacteroides thetaiotaomicron. J Bacteriol. 1983 Apr;154(1):287–293. doi: 10.1128/jb.154.1.287-293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Oyaizu Y., Oyaizu H., Woese C. R. Natural relationship between bacteroides and flavobacteria. J Bacteriol. 1985 Oct;164(1):230–236. doi: 10.1128/jb.164.1.230-236.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]


