Abstract
Most proteins present in chloroplasts are synthesized in the cytosol and are posttranslationally translocated into the organelle. A multicomponent translocation machinery located in both the outer and the inner envelope of chloroplasts was identified, but the mode of action of many subunits remains unclear. Here, we describe the regulation of an early step of translocation occurring at the outer envelope. The outer envelope translocon subunit Toc34 can be phosphorylated, and GTP binding is regulated by phosphorylation. In vitro, Toc34 acts as a receptor for proteins containing a chloroplast-targeting signal. Interaction of Toc34 with the transit peptide is highly regulated and depends on GTP binding to Toc34 and on phosphorylation of the transit peptide of the preprotein.
Keywords: GTP binding, preprotein recognition, chloroplast import, pea, envelope
Proteins are translocated over the plastid membranes by a proteinaceous import machinery (1–3). The general process of translocation involves the cytosolic sorting of proteins, which have to migrate toward the target membrane, the recognition at the surface of the organelle, and the final hand-over to the translocon. Although the general principles of protein translocation across membranes of different organelles are similar, each translocation machinery reveals unique features. For example, the import apparatus of the two endosymbiotically derived organelles (4), mitochondria and chloroplasts, share the same general structure (3, 5). However, none of the outer mitochondrial membrane proteins identified to be involved in translocation of preproteins were found to require the interaction with nucleotides. Furthermore, transport of the preproteins from the receptor to the general insertion pore is energy-independent (5). This is distinct from the requirements of protein import into chloroplasts, where two of the subunits of the outer envelope, namely Toc160 (6, 7) and Toc34 (34-kDa translocon at the outer envelope of chloroplast) (8, 9), interact with nucleotides. In addition, the translocation processes at the outer envelope depend on the hydrolysis of nucleotides (10–14). Further, a cycle of phosphorylation/dephosphorylation of the chloroplast transit sequence regulates its insertion in contrast to the mitochondrial precursor, which will not be modified (2, 5, 15, 16).
Here, we have investigated Toc34 to study the regulation of a single step in the overall translocation process. Toc34 is in close proximity to the preprotein during import (8) and is associated with the translocation pore Toc75 (9). Toc34 contains a single transmembrane domain at the C terminus, whereas the N-terminal domain is exposed to the cytosol. The GTP-binding domain of Toc34 is homologous to the GTP-binding side found in the C-terminal part of Toc160 (8, 9, 17), a further Toc subunit with distinct transit-peptide recognition function (7, 17, 18).
The GTP-binding property of Toc34 might enable the protein to regulate the translocation process by interaction with Toc160 and Toc75 (9), or it might be a receptor for preproteins (8, 18), or both. The current evidence would fit both ideas because only crosslinks, but no direct interactions between preproteins and Toc34, have been shown (18). Crosslinking might be a result of close proximity but not of a direct interaction between both partners.
Here, we present evidence for the physical interaction between Toc34 and preSSU (precursor of the small subunit of ribulose 1,5 biphosphate carboxylase-oxygenase) in vitro in its GTP-bound form, but not in its GDP-bound state. This interaction is regulated in a complex interplay of nucleotide binding and protein phosphorylation. Toc34 can be phosphorylated by a protein kinase present in the outer envelope. This phosphorylation decreases the affinity of Toc34 for GTP and regulates the GTP-dependent interaction with the precursor protein. The interaction between Toc34 and the preprotein is strongly enhanced by phosphorylation of the transit peptide.
Materials and Methods
General.
The bacterial strain BL21-DE3 and the overexpression vector pET21d were obtained from Calbiochem–Novabiochem (Bad Soden, Germany). Dodecylmaltoside was purchased from Roche Molecular Biochemicals. [α-32P]ATP, [α-32P]GTP, [γ-32P]ATP, and [γ-32P]GTP were from Amersham Pharmacia. All other chemicals used were purchased from Roth (Karlsruhe, Germany) or Sigma.
Standard procedures, like the purification of the outer envelope of chloroplasts or immunoprecipitation of Toc34, have been described (9). A protein kinase-containing fraction was partially purified from wheat germ, as described in ref. 15.
Construction of Toc34ΔTM252–6His.
The pET21d-Toc34 plasmid (9) was used as template in standard PCR with the T7-promotor primer and a C-terminal primer containing an XhoI cleavage side after amino acid 252. The resulting PCR fragment was cloned into the NcoI/XhoI site of pET21d.
Expression and Purification of Toc34ΔTM252–6His.
Escherichia coli BL21-DE3 harboring the plasmid pET21d-Toc34ΔTM252–6His were grown in M9ZB containing 25 μg/ml ampicillin at 37°C and induced with 1 mM isopropyl-β-d-thiogalactopyranoside at an absorption of A600 = 0.8. Cells were harvested after 3 h by centrifugation, and pellets were stored at −70°C. Cells containing Toc34ΔTM252–6His were resuspended in buffer A (100 mM Na-phosphate, pH 7.0/10 mM Tris/100 mM NaCl/10 mM β-mercaptoethanol/10 mM MgCl2/10% glycerol/0.5% Triton X-100) at a concentration of 0.2 mg/ml (wet cell pellet). Cells were lysed by using the French pressure cell, sonicated on ice for 30 s at 200 W, incubated with DNase I (5 μg/ml final concentration) for 30 min, and centrifuged at 10,000 × g at 4°C for 20 min.
The supernatant (10 ml) was loaded onto a 1.5-ml Talon-Metal-Affinity Column (CLONTECH) equilibrated with buffer A. The resin was washed with 7.5 ml of buffer A followed by a step gradient of 200, 300, and 400 mM NaCl in 100 mM Na-phosphate, pH 6.6/20 mM Tris/5 mM β-mercaptoethanol/1 mM imidazol/10% glycerol. Toc34 was eluted in 100 mM Na-phosphate, pH 5.6/20 mM Tris/500 mM NaCl/5 mM β-mercaptoethanol/50 mM imidazol/10% glycerol. The protein was dialysed against buffer, as indicated for every experiment.
Binding of GTP to Toc34.
Purified outer envelopes or Toc34ΔTM252–6His in 20 mM Tris, pH 7.5/120 mM NaCl were incubated with 10 nM [α-32P]GTP (3,000 Ci/mmol) for 15 min at 4°C in 50 μl final volume. After incubation, the interaction was fixed by addition of sodium periodat (5 mM final concentration). After 4 min, sodium cyanoborohydride (5 mM final concentration) was added (19). Finally, the proteins were separated by SDS/PAGE, and labeling was visualized by autoradiography.
Phosphorylation of Toc34.
Purified outer envelopes or Toc34ΔTM252–6His in 20 mM Tris, pH 7.5/120 mM NaCl/5 mM MgCl2/0.5 mM MnCl2 were incubated for 10 min at room temperature or at 4°C (as indicated) in 50 μl final volume with 10 nM [γ-32P]GTP (3,000 Ci/mmol) or [γ-32P]ATP (5,000 Ci/mmol). Proteins were subjected to SDS/PAGE without further treatment, and phosphorylation was visualized by autoradiography. Phosphorylated Toc34 was excised from the gel and treated with endoproteinase Glu-C protease as described in refs. 20 and 21.
Immunoprecipitation of the preSSU–Toc34 Complex.
preSSU was overexpressed, purified, and phosphorylated as described (15). The phosphorylated protein was passed over a Talon-Metal-Affinity Column (CLONTECH). Labeled fractions were analyzed by TLC on polyethyleneimine-cellulose plates (22) for the absence of radioactive ATP or phosphate. Finally, various amounts of phosphorylated [32P]preSSU were incubated with an indicated amount of Toc34ΔTM252–6His for 15 min at 22°C in 20 mM Tris, pH 7.5/120 mM NaCl/1.25 μM ATP/5 mM MgCl2 in the presence of 1 mM GTP if not indicated otherwise. Binding was determined by coimmunoprecipitation with protein A-Sepharose (25 μl; Amersham Pharmacia) preloaded with Toc34 antibodies. After incubation, the matrix was washed twice with immunoprecipitation buffer (20 mM Tris, pH 7.5/120 mM NaCl) containing 1% NP-40 and three times without detergent. The protein was eluted by adding SDS-sample buffer and boiling at 95°C for 3 min.
Quantification of the Radioactivity.
Phosphorylation or GTP binding of Toc34ΔTM252–6His or outer envelope Toc34 was quantified by dissolving the dried SDS/PAGE gel slices in 30% H2O2 and 60% HClO4 for 16 h at 60°C followed by scintillation counting. Data were presented by using sigma plot 5.0 (SPSS, Chicago). The binding between Toc34 and preSSU was analyzed by Skatchard plot analysis (23).
Results
Expression and Purification of Toc34ΔTM252.
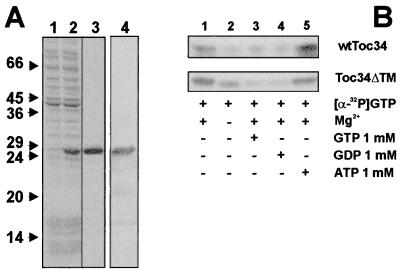
Toc34 is a chloroplastic outer envelope protein containing a single transmembrane domain at its C terminus (8, 9). To express the protein in a soluble form and to avoid denaturation during purification (9, 24), the cDNA coding for Toc34 was truncated, resulting in expression of the cytosolic nucleotide binding domain of Toc34 only. Toc34ΔTM252–6His was synthesized with high yields after induction with isopropyl-β-d-thiogalactopyranoside (Fig. 1A, compare lanes 1 and 2). The soluble protein was purified close to homogeneity by affinity chromatography on a Talon-metal matrix (Fig. 1A, lane 3). The purified polypeptide was identified as the truncated form of Toc34 protein by immunoreaction with antibodies (Fig. 1A, lane 4) raised against the full-length protein (9).
Figure 1.
Expression, purification, and functional analysis of Toc34ΔTM252. The protein was expressed as described in Materials and Methods. (A) Total bacterial extract before (lane 1) and after (lane 2) induction of expression, and the fraction after completed purification (lanes 3 and 4) was subjected to SDS/PAGE followed by Coomassie blue staining (lanes 1–3) or immunodetection using αToc34 antibodies (lane 4). Numbers indicate the molecular mass standard in kDa. (B) A total of 25 μg of either outer envelope protein or 2.5 μg purified Toc34ΔTM252–6His was incubated with [α-32P]GTP at 4°C in the presence (lanes 1, 3–5) or absence (lane 2) of 5 mM MgCl2 and in the presence of 1 mM of either GTP (lane 3), GDP (lane 4), or ATP (lane 5).
To demonstrate a functional similarity between Toc34ΔTM252–6His and wtToc34 present in outer envelope, we compared the GTP-binding ability of both proteins (8, 9). Both proteins recognized GTP in the presence of Mg2+ with higher efficiency, as in the absence of Mg2+ (Fig. 1B, lanes 1 and 2). Binding of radioactive-labeled GTP could be competed by an excess of unlabeled GTP or GDP (Fig. 1B, lanes 3 and 4), but not by ATP (Fig. 1B, lane 5), demonstrating the specificity of both proteins for GTP. The stimulation of GTP binding of wtToc34 by 1 mM ATP (Fig. 1B, lane 5) was seen before (9) and will be discussed in a later section.
Phosphorylation of Toc34.
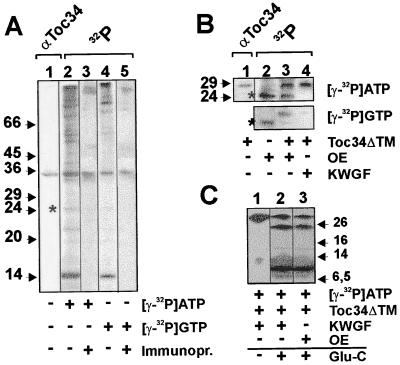
The outer envelope contains distinct GTP and ATP kinases that phosphorylate different proteins of the outer envelope, for example, a 32.5-kDa protein (value extrapolated from running behavior on SDS/PAGE in ref. 25). We asked whether this 32-kDa protein might be Toc34. We observed the same phosphorylation pattern as described (compare in ref. 25, Fig. 2, lanes 11 and 12 and this report, Fig. 2A, lanes 2 and 4). Immunodetection and immunoprecipitation with Toc34 antibodies revealed that Toc34 was indeed phosphorylated by both ATP and GTP (Fig. 2A, lanes 3 and 5). We conclude that the earlier described 32.5-kDa protein (25) is Toc34. Toc34ΔTM252–6His also was phosphorylated with high efficiency independent of the type of nucleotide present when incubated with outer envelope membranes (Fig. 2B, lane 3). The phosphorylated 24-kDa protein (∗) is an unidentified protein in the outer envelope membrane (compare Fig. 2A, lane 2 and Fig. 2B, lane 2) and preferentially phosphorylated by an ATP-dependent kinase. Toc34ΔTM255–6His also could be phosphorylated by a partially purified protein kinase fraction from wheat germ (Fig. 2B, lane 4). Phosphorylation by wheat germ extract (15) is nucleotide-specific for ATP. Limited proteolysis (20) of the Toc34ΔTM255–6His phosphorylated either by the kinase present in the outer envelope or by the kinase-enriched wheat germ fraction using endoproteinase Glu-C revealed an identical pattern of labeled peptides (Fig. 2C, lanes 2 and 3), suggesting that both kinases phosphorylate the same sites of Toc34.
Figure 2.
Toc34 can be phosphorylated with ATP and GTP by a kinase present in the outer envelope or by a kinase-enriched fraction from wheat germ lysate. (A) Outer envelope membranes were subjected to SDS/PAGE and immunodecorated with αToc34 antibodies (lane 1). Outer envelope membranes (20 μg of total protein) were incubated with [γ-32P]ATP (lanes 2 and 3) or [γ-32P]GTP (lanes 4 and 5) and analyzed on SDS/PAGE before (lanes 2 and 4) and after immunoprecipitation using αToc34 antibody (lanes 3 and 5). (B) Outer envelopes (10 μg of total protein; OE) were incubated with γ-32P-labeled nucleotides in the absence (lane 2) or presence (lane 3) of 2 μg of purified Toc34ΔTM252–6His. The immunodecoration of the mixture using αToc34 antibodies is shown in lane 1 for identification of Toc34ΔTM252–6His. In lane 4, purified Toc34ΔTM252–6His (2 μg) and 0.5 ng of a protein kinase-enriched wheat germ fraction (KWGF) (15) were incubated with [γ-32P]ATP or [γ-32P]GTP. (A and B) A phosphorylated unknown 24-kDa protein is indicated for comparison of labeling efficiency (*). (C) Toc34ΔTM252–6His phosphorylated by the protein kinase-enriched wheat germ fraction (lanes 1 and 2, KWGF) or a kinase present in the outer envelope (lane 3) was cleaved by using endoproteinase Glu-C. Arrows and numbers in A–C indicate the molecular mass standard in kDa.
GTP Binding of Toc34 Is Regulated by Phosphorylation.
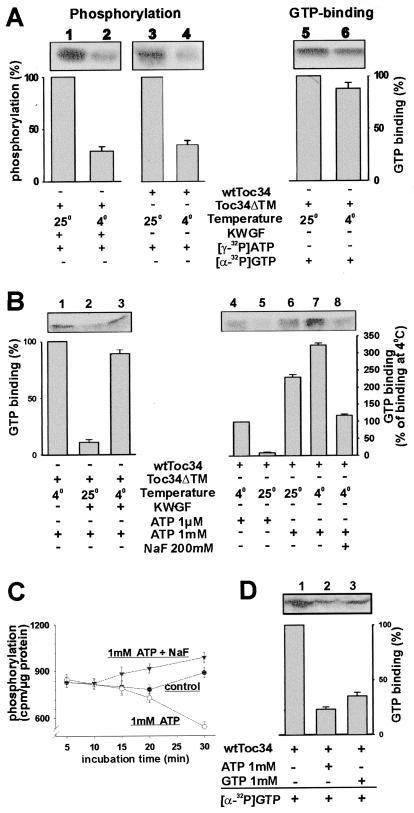
To investigate the effect of phosphorylation on GTP binding of Toc34, we tried to experimentally separate both processes. Whereas the binding of GTP to Toc34 was hardly altered in the range between 4°C and 25°C (Fig. 3A, compare lanes 5 and 6, and data not shown), the phosphorylation dropped by a factor of four when the temperature was shifted from 25°C to 4°C (Fig. 3A). This difference in temperature sensitivity between phosphorylation and GTP binding was used as a tool to study the role of phosphorylation on GTP binding. The binding of GTP to Toc34ΔTM255–6His was reduced drastically at 25°C in the presence of ATP and the protein kinase-enriched wheat germ fraction (Fig. 3B, lanes 1 and 2), but was not altered by the presence of ATP only (see Fig. 1B Lower, lanes 1 and 5, and data not shown). To distinguish whether GTP binding was affected by the phosphorylation of Toc34 or by the presence of the kinase fraction, the GTP-binding experiment was performed at 4°C, where phosphorylation is strongly reduced (Fig. 3A, lanes 1 and 2). The presence of the kinase fraction did not alter the ability of the overexpressed Toc34ΔTM255–6His to recognize GTP at 4°C (Fig. 3B, lanes 1 and 3). We conclude that the reduction of GTP binding depends on the phosphorylation of Toc34. We then incubated outer envelopes with GTP at 4°C and 25°C, respectively (Fig. 3B, lanes 4–8) to test whether wtToc34 present in outer envelopes has the same properties as the overexpressed protein. GTP binding was almost abolished in the presence of 1 μM ATP at 25°C, where phosphorylation occurred, but not at 4°C (Fig. 3B, lane 4 and 5).
Figure 3.
Phosphorylation of Toc34 is temperature-dependent and regulates GTP binding to Toc34. (A) wtToc34 (lanes 3 and 4) was phosphorylated by a protein kinase present in outer envelopes and Toc34ΔTM252–6His (2 μg; lanes 1 and 2) by a protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF) in the presence of [γ-32P]ATP at 25°C (lanes 1 and 3) or 4°C (lanes 2 and 4). Equal amounts of Toc34ΔTM252–6His were incubated with [α-32P]GTP at 25°C (lane 5) or 4°C (lane 6), and GTP binding was determined. (B) A total of 2 μg of purified Toc34ΔTM252–6His was incubated for 20 min with [α-32P]GTP and 1 mM ATP in the absence (lane 1) or presence (lanes 2 and 3) of the protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF) at 4°C (lanes 1 and 3) or 25°C (lane 2), and binding of GTP was determined. A total of 20 μg of outer envelope protein was incubated for 20 min with [α-32P]GTP in the presence of 1 μM ATP (lanes 4 and 5) or 1 mM ATP (lanes 6–8) at 25°C (lanes 5 and 6) or 4°C (lanes 4, 7, and 8). In lane 7, 200 mM of NaF was added. (C) Phosphorylation of wtToc34 present in outer envelopes by [γ-32P]ATP (●) in the presence of 1 mM ATP (○) or 1 mM ATP and 200 mM NaF (▾) was determined at different incubation times. Each point represents the average of at least two independent experiments. (D) Outer envelopes were phosphorylated for 5 min with either 1 mM ATP (lane 2) or 1 mM GTP (lane 3), washed twice without nucleotides and then incubated with [α-32P]GTP. (A, B, and D) The averaged results of at least three independent experiments are shown as histogram. (A) The observed phosphorylation or GTP binding at 25°C (lanes 1, 3, and 5); (B) the GTP binding at 4°C (lanes 1 and 4); and (D) the GTP binding without phosphorylation was set to 100%.
The earlier experiments had shown that GTP binding of Toc34 increases in the presence of 1 mM ATP (Fig. 1B, lane 5, and ref. 9). This effect is not seen for Toc34ΔTM255–6His (Fig. 1B, lane 5) or at 1 μM ATP (Fig. 3B, lane 5). One possible explanation for this effect could be that Toc34 might be dephosphorylated in an ATP-dependent manner by a phosphatase present in the outer envelope membranes. Consequently, addition of higher amounts of ATP would result in dephosphorylation and therefore in a stimulation of GTP binding. Phosphorylation of wtToc34 by low amounts of ATP occurred rapidly and remained stable for 30 min (Fig. 3C, control). In the presence of 1 mM ATP, the amount of phosphorylated protein was drastically reduced after 30 min (Fig. 3C, 1 mM ATP). In contrast, when NaF was used to block the phosphatase activity (26), no reduction but a slight increase of phosphorylated Toc34 was observed (Fig. 3C, 1 mM ATP + NaF). This supports the hypothesis that an ATP-dependent phosphatase is present in the outer envelope.
The increase of GTP binding by wtToc34 after addition of ATP did not depend on the temperature (Fig. 3B, lanes 6 and 7) but on the concentration of ATP (Fig. 3B, lanes 5 and 6), leading to the interpretation that the Km for ATP of the phosphatase is significantly higher then the Km of the kinase. The phosphatase might be involved in regulation of the nucleotide binding of Toc34, because addition of NaF at 4°C and 1 mM ATP resulted in the same GTP-binding efficiency as in the presence of 1 μM ATP (Fig. 3B, lanes 4 and 8). The reduction of GTP interaction was not an effect of the increased salt concentration, because an increase of the NaCl concentration to 200 mM did not influence the specific interaction of Toc34 with GTP (data not shown). Therefore, Toc34 present in purified outer envelope membranes already might be partially phosphorylated, when isolated from intact chloroplasts. ATP-dependent dephosphorylation in our experiments then would result in an increase of GTP binding. We conclude that a cycle of phosphorylation and dephosphorylation of Toc34 regulates the GTP-binding properties.
Interestingly, the in vitro inhibition of GTP binding of wtToc34 can be observed by phosphorylation in an ATP- and GTP-dependent manner (Fig. 3D, lanes 1–3).
Toc34 Complexed with GTP Recognizes Specifically Phosphorylated Preprotein.
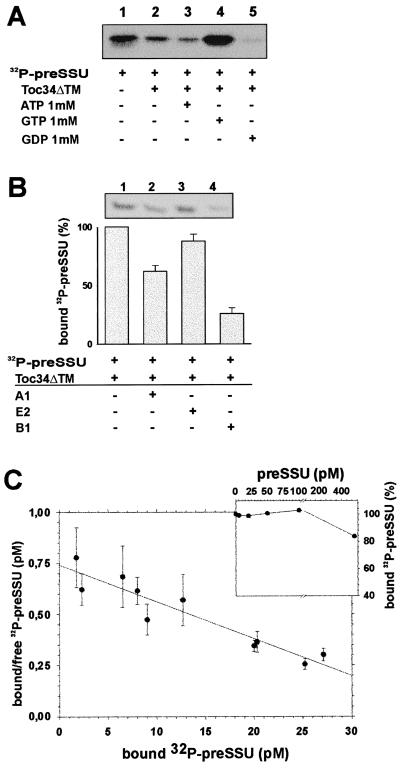
So far, the results indicate that binding of GTP to Toc34 is regulated by phosphorylation. However, it is still unknown which process might be regulated by the GTP-binding cycle. Toc34 was shown to crosslink to the preprotein preSSU (18). Therefore, Toc34 might act as a receptor. Different precursor proteins are phosphorylated within their targeting sequence (15), one of which, [32P]preSSU, was used to investigate whether Toc34 binds to preproteins. As shown in Fig. 4A, Toc34ΔTM255–6His interacts with [32P]preSSU (lane 2), and this interaction is drastically enhanced in the presence of GTP (lane 4) but strongly decreased in the presence of GDP (lane 5). The mature protein SSU is not recognized by Toc34 (not shown). Because Toc34 is a membrane-anchored protein, the structure of the cytosolic domain might be influenced by the lipid surface. To mimic the hydrophobic environment, the above experiment was repeated in the presence of 5 mM dodecylmaltoside, a detergent that was found to stabilize the Toc complex after isolation (E.S., unpublished observation), but no difference in the binding behavior could be observed (data not shown).
Figure 4.
Toc34 interacts specifically with the phosphorylated precursor protein preSSU. (A) A total of 20 pM of [32P]preSSU (25% shown in lane 1) was incubated with 10 nM of Toc34ΔTM252–6His (lanes 2–5) in the presence of 1 mM ATP (lane 3), 1 mM GTP (lane 4), and 1 mM GDP (lane 5), and the complex was immunoprecipitated by using αToc34 antibodies. (B) A total of 20 pM [32P]preSSU were incubated with 10 nM Toc34ΔTM252–6His in the presence of 1 mM GTP (lane 1). After 10 min, 250 nM of nonphosphorylated peptide A1 (MVAPFTGLKSAASFPVSRKQNLDITS; lane 2), E2 (MASSVLSSAAVATRSN VAQAN; lane 3), or 250 nM phosphorylated peptide B1 (MVAPFTGLKSA AS*FPVSRKQNLDITS; lane 4) was added, and the remaining complex was immunoprecipitated. Histogram represents the average of at least three independent experiments, where [32P]preSSU binding to Toc34ΔTM252–6His in the absence of competitor was set to 100%. (C) A total of 0.1 nM Toc34ΔTM252–6His was incubated with different amounts of [32P]preSSU in the presence of 1 mM GTP, the complex was immunoprecipitated, and the binding was quantified. The average of three independent experiments is shown as Skatchard plot, resulting in a dissociation constant of Kd = 0.10 ± 0.04 nM; C Inset) A total of 0.1 nM Toc34ΔTM252–6His was incubated with 10 pM (●) [32P]preSSU in presence of 1 mM GTP and of different amounts of nonphosphorylated preSSU. Values are expressed as percent [32P]preSSU bound without competition with nonphosphorylated preSSU.
To address the question whether phosphorylation of the preprotein alters the interaction with Toc34, different peptides representing different parts of the preSSU transit peptide were tested to compete against the interaction between Toc34 and [32P]preSSU. The peptides A1 and B1 are identical, except that B1 is phosphorylated. The peptide E2 represents a C-proximal portion of the preSSU-targeting signal. Addition of the nonphosphorylated peptides E2 and A1 had only a minor effect on the interaction between Toc34ΔTM255–6His and [32P]preSSU (Fig. 4B). However, the same concentration of phosphorylated peptide B1 was able to largely disrupt the complex (Fig. 4B, compare lanes 1 and 4). To exclude that the mature portion of the preprotein influences the binding behavior of the phosphorylated precursor to Toc34, competition experiments were performed in the presence of heterologous preSSU. Fig. 4C Inset shows that the presence of a 5-fold excess of nonphosphorylated preSSU does not disrupt the complex between Toc34ΔTM255–6His and [32P]preSSU.
Next, we investigated the affinity between Toc34ΔTM255–6His and [32P]preSSU in the presence of GTP and observed a dissociation constant of Kd = 0.1 nM (Fig. 4C). This dissociation constant is about 3 orders of magnitude lower than the dissociation constant described for the mitochondrial preprotein receptor humanTom20 and preornithyl carbamyltransferase (27). This suggests a nucleotide or phosphorylation-regulated release of the preprotein from the Toc34 protein.
The Interaction Between Toc34 and a Precursor Protein Is Regulated by GTP/GDP Binding and Phosphorylation.
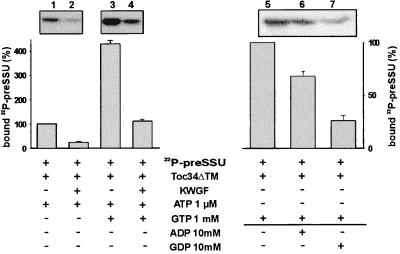
GTP induces the high-affinity interaction between nonphosphorylated Toc34ΔTM255–6His and phosphorylated preSSU (Fig. 4A). Further, the GTP binding is regulated by phosphorylation (Fig. 3). We therefore asked whether the recognition of preSSU by Toc34 is influenced by phosphorylation as well. Binding of [32P]preSSU to phosphorylated Toc34ΔTM255–6His was at least 4-fold decreased in comparison to the binding to nonphosphorylated Toc34. This decrease did not depend on the presence of GTP (Fig. 5A, compare lanes 1 and 2 with lanes 3 and 4). However, when phosphorylated Toc34 was incubated with GTP, binding of [32P]preSSU was higher than in the absence of GTP. This is probably because of incomplete phosphorylation of Toc34ΔTM255–6His in vitro (Fig. 5A, compare lanes 2 and 4).
Figure 5.
Toc34 interaction with preSSU is regulated by phosphorylation and a GTP/GDP cycle. A total of 20 pM [32P]preSSU was incubated with 10 nM Toc34ΔTM252–6His (lanes 1–4) and 1 μM ATP in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 1 mM GTP, and in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of a protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF); complex formation was tested by immunoprecipitation. (B) A total of 20 pM of [32P]preSSU was incubated with 10 nM Toc34ΔTM252–6His in the presence of 1 mM GTP (lane 1). After 10 min of preincubation, 10 mM of ADP (lane 2) or GDP (lane 3) was added, and the remaining complex was immunoprecipitated. Histograms represent averaged value of three independent experiments. (A) Binding to nonphosphorylated Toc34 was set to 100%; (B) binding without competition was set to 100%.
[32P]preSSU will not bind to Toc34 in the presence of GDP (Fig. 4A, lane 5). We wanted to assess the influence of a GTP/GDP exchange on the preSSU–Toc34 complex. Complex formation was initiated under GTP conditions (Fig. 5, lane 5). GTP exchange was induced by the addition of 10 mM GDP or ADP, respectively. Although the addition of GDP induced a large release of the preprotein from Toc34, ADP had only a slight effect (Fig. 5, lane 6 and 7). We conclude that a GTP/GDP cycle can regulate precursor binding and release from Toc34.
Discussion
Major components of the translocation machinery for protein import into chloroplasts have been identified. It is now important to define the function of the individual components and to gain insights into their mode of action. Therefore, we overexpressed Toc34 with a deletion of the transmembrane domain, which resulted in the expression of a soluble polypeptide. The overexpressed protein has similar GTP-binding properties as the wild-type protein in situ (Fig. 1). Toc34 has a specific binding site for guanosine nucleotides within the cytosolic domain because the interaction with GTP was competed for by GTP and GDP, but not by ATP. We did not observe a GTPase activity of Toc34 under the conditions used (N.S. and E.S., unpublished observations). This result leads to two possible explanations: Toc34 could be regulated by GTP/GDP exchange or binding, but not by the hydrolysis of GTP. Alternatively, a cytosolic or membrane-located cofactor could stimulate GTP hydrolysis (28–30), but was absent in the reconstituted experiments described here.
Earlier reports have demonstrated that not only GTP binding by the receptor components but also the phosphorylation of the transit peptide influenced the import reaction (14, 15, 17). Further, many proteins of the outer envelope are phosphorylated by protein kinases present in the envelope (25), one of them being Toc34 (Fig. 2). The overexpressed Toc34ΔTM255–6His also is phosphorylated by an envelope-attached kinase in an ATP- or GTP-dependent manner. A soluble protein kinase fraction from wheat germ could phosphorylate Toc34 with high efficiency, but only in the presence of ATP (Fig. 2). The wheat germ fraction most likely contains more then one active kinase (T. May and J.S., unpublished observations) but could be used as a tool in this study, because we observed an identical labeled fragmentation pattern of Toc34 phosphorylated either by the kinase present in the outer envelope or in the wheat germ fraction (Fig. 2).
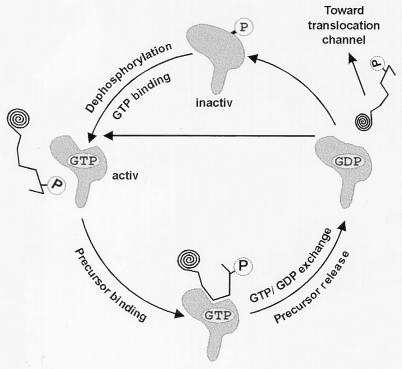
In general, phosphorylation regulates protein action, interaction, or degradation (31–33). As indicated here, the phosphorylation of Toc34 regulates the binding of GTP (Fig. 3). The interaction between the translocation complex subunit Toc34 and GTP might have two distinct regulative functions: (i) it could influence the stability of the import complex, or (ii) it could regulate the interaction with preproteins. The first function is hard to determine because only three proteins of the outer envelope have been identified so far as being involved in translocation (2, 3), and other still unidentified components are expected. Using the three known proteins as marker, no clear dependence of the stability of the Toc complex on the concentration of GTP was observed (E.S., unpublished observation). For the later function, we first had to demonstrate that Toc34 physically interacts with a preprotein. Several crosslinking studies (8, 18) provided initial evidence that Toc34 might recognize preproteins, however, it could not be excluded that this was because of the close proximity between the preprotein and Toc34. Here, we show that Toc34 acts as a receptor by specific interaction with the transit peptide (Fig. 4). The affinity of the receptor is drastically increased in the presence of GTP. Therefore, the binding of GTP might be necessary in vivo for interaction between Toc34 and the transit peptide. However, crosslinks between Toc34 and the transit peptide were achieved only in the absence of hydrolysable nucleotides when intact chloroplasts were used (18). This finding might be explained by a nucleotide-stimulated release of the precursor from Toc34 because the transfer of the preprotein from Toc34 into the translocation pore was found to be GTP-dependent (14). Further, the nucleotide dependence of Toc160 and Toc34 cannot be separated in the performed in situ experiments (18), and the presence of the ATP- and GTP-dependent preprotein receptor Toc160 might influence the preprotein release from Toc34 in a nucleotide-dependent manner (for review see ref. 2). Therefore, the absence of hydrolysable nucleotides might be essential to rescue the transit peptide on the receptor Toc34 in situ. The low binding affinity observed in the absence of nucleotides (ref. 18 and Fig. 4) is consistent with this interpretation. In contrast to the low binding affinity in the absence of GTP, the affinity for the phosphorylated preprotein was found to be very high (Kd = 0.1 nM) in the presence of 1 mM GTP. Even although Toc34 can recognize preSSU in a nonphosphorylated state, the affinity was found to be lower than for the phosphorylated protein (Fig. 4 Inset). In addition, only a phosphorylated targeting signal peptide was able to dissociate the Toc34 [32P]preSSU complex (Fig. 4). Phosphorylated Toc34 can neither recognize the preprotein nor bind GTP. Dephosphorylation of Toc34 is achieved by a phosphatase in an ATP-dependent manner (Fig. 3). The complexed protein will be released in the presence of GDP (Fig. 5). All results together lead to a complex mechanism of regulation of Toc34, as presented in Fig. 6.
Figure 6.
Model of preprotein recognition by Toc34. See text for detailed discussion.
We conclude that phosphorylation inactivates the receptor function of Toc34 at two levels. First, GTP binding is inhibited, and therefore the affinity of Toc34 to preproteins is decreased. Second, the binding of the preprotein itself is blocked (Fig. 5). Dephosphorylation activates the function of Toc34 as a receptor. We postulate that Toc34 after dephosphorylation will first bind to GTP and subsequently form a high affinity complex with the phosphorylated precursor. The precursor then will be released either by GTP/GDP exchange or by GTP hydrolysis. Whether Toc34 acts before or after the precursor receptor Toc160 (7, 17, 18) remains to be investigated. However, the preprotein has to be dephosphorylated after release from Toc34 and before entering the translocation channel Toc75.
The identification of Toc34 as a phosphorylation and GTP/GDP-regulated receptor is a step in the understanding of the “handing over process” involved in the transport of preproteins toward the translocon.
Acknowledgments
We thank T. May for the kinase-enriched wheat germ fraction. The research was supported by grants from the Deutsche Forschungsgemeinschaft, the Human Frontier Science Program, and Fonds der Chemischen Industrie. N.S. was a recipient of a stipendium of the Graduierten Kolleg.
Abbreviations
- Toc34
34-kDa translocon at the outer envelope of chloroplast
- preSSU
precursor of the small subunit of ribulose 1,5 biphosphate carboxylase-oxygenase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080491597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080491597
References
- 1.Schnell D, Blobel G, Keegstra K, Kessler F, Ko K, Soll J. Trends Cell Biol. 1997;7:303–304. doi: 10.1016/S0962-8924(97)01111-2. [DOI] [PubMed] [Google Scholar]
- 2.Heins L, Collinson I, Soll J. Trends Plant Sci. 1998;3:56–61. [Google Scholar]
- 3.Keegstra K, Froehlich J E. Curr Opin Plant Biol. 1999;2:471–476. doi: 10.1016/s1369-5266(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 4.Schatz G, Dobberstein B. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 5.Neupert W. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 6.Bölter B, May T, Soll J. FEBS Lett. 1998;441:59–62. doi: 10.1016/s0014-5793(98)01525-7. [DOI] [PubMed] [Google Scholar]
- 7.Bauer J, Chen K, Hiltbrunner A, Wherli E, Eugster M, Schnell D, Kessler F. Nature (London) 2000;403:203–207. doi: 10.1038/35003214. [DOI] [PubMed] [Google Scholar]
- 8.Kessler F, Blobel G, Patel H A, Schnell D J. Science. 1994;266:1035–1039. doi: 10.1126/science.7973656. [DOI] [PubMed] [Google Scholar]
- 9.Seedorf M, Waegemann K, Soll J. Plant J. 1995;7:401–411. doi: 10.1046/j.1365-313x.1995.7030401.x. [DOI] [PubMed] [Google Scholar]
- 10.Flügge U I, Hinz G. Eur J Biochem. 1986;160:563–570. doi: 10.1111/j.1432-1033.1986.tb10075.x. [DOI] [PubMed] [Google Scholar]
- 11.Schindler C, Hracky R, Soll J. Z Naturforsch. 1987;42:103–108. [Google Scholar]
- 12.Olsen L J, Keegstra K. J Biol Chem. 1989;264:6724–6729. [PubMed] [Google Scholar]
- 13.Olsen L J, Keegstra K. J Biol Chem. 1992;267:433–439. [PubMed] [Google Scholar]
- 14.Young M E, Keegstra K, Froehlich J E. Plant Physiol. 1999;121:237–243. doi: 10.1104/pp.121.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waegemann K, Soll J. J Biol Chem. 1996;271:6545–6554. doi: 10.1074/jbc.271.11.6545. [DOI] [PubMed] [Google Scholar]
- 16.May T, Soll J. Plant Cell. 2000;11:1–12. doi: 10.1105/tpc.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J. Science. 1994;266:1989–1992. doi: 10.1126/science.7801125. [DOI] [PubMed] [Google Scholar]
- 18.Kouranov A, Schnell D J. J Cell Biol. 1997;139:1677–1685. doi: 10.1083/jcb.139.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peter M E, She J, Huber L A, Terhorst C. Anal Biochem. 1993;210:77–85. doi: 10.1006/abio.1993.1153. [DOI] [PubMed] [Google Scholar]
- 20.Cleveland D W, Fischer S G, Kirschner M W, Laemmli U K. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- 21.Soll J, Bennett J. Eur J Biochem. 1988;175:301–307. doi: 10.1111/j.1432-1033.1988.tb14197.x. [DOI] [PubMed] [Google Scholar]
- 22.Trifilo R M, Dobson J G. J Chromatogr. 1976;116:465–467. doi: 10.1016/s0021-9673(00)89919-7. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz G. Biophys Struct Mech. 1976;2:1–12. doi: 10.1007/BF00535648. [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Schnell D J. J Biol Chem. 1997;272:6614–6620. doi: 10.1074/jbc.272.10.6614. [DOI] [PubMed] [Google Scholar]
- 25.Soll J, Fischer I, Keegstra K. Planta. 1988;176:488–496. doi: 10.1007/BF00397655. [DOI] [PubMed] [Google Scholar]
- 26.Bennett J. Eur J Biochem. 1980;104:85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- 27.Schleiff E, Turnbull J L. Biochemistry. 1998;37:13043–13051. doi: 10.1021/bi9807456. [DOI] [PubMed] [Google Scholar]
- 28.Bourne H R, Sanders D A, McCormick F. Nature (London) 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 29.Scheffzek K, Ahmadian M R, Wittinghofer A. Trends Biochem Sci. 1998;23:257–262. doi: 10.1016/s0968-0004(98)01224-9. [DOI] [PubMed] [Google Scholar]
- 30.de Vries L, Farquhar M G. Trends Cell Biol. 1999;9:138–144. doi: 10.1016/s0962-8924(99)01515-9. [DOI] [PubMed] [Google Scholar]
- 31.Staehelin L A, Arntzen C J. J Cell Biol. 1983;97:1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKintosh C. Curr Opin Plant Biol. 1998;1:224–229. doi: 10.1016/s1369-5266(98)80108-8. [DOI] [PubMed] [Google Scholar]
- 33.Schenk P W, Snaar-Jagalska B E. Biochim Biophys Acta. 1999;1449:1–24. doi: 10.1016/s0167-4889(98)00178-5. [DOI] [PubMed] [Google Scholar]