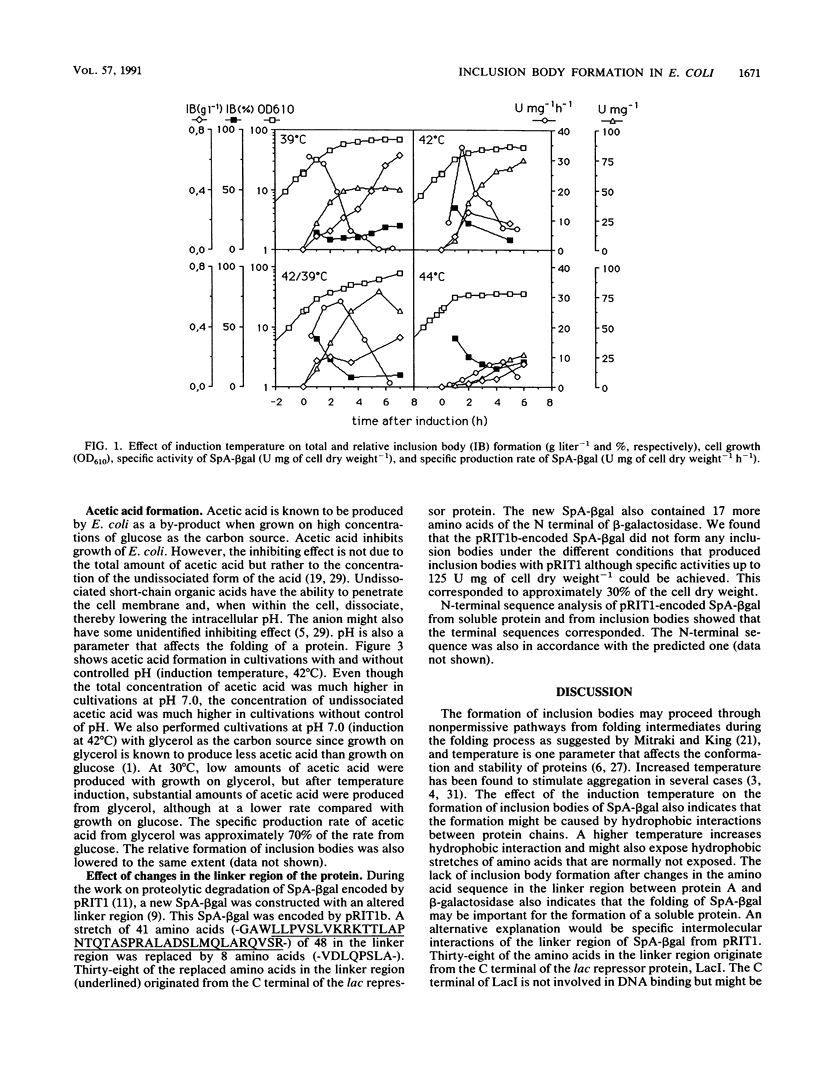
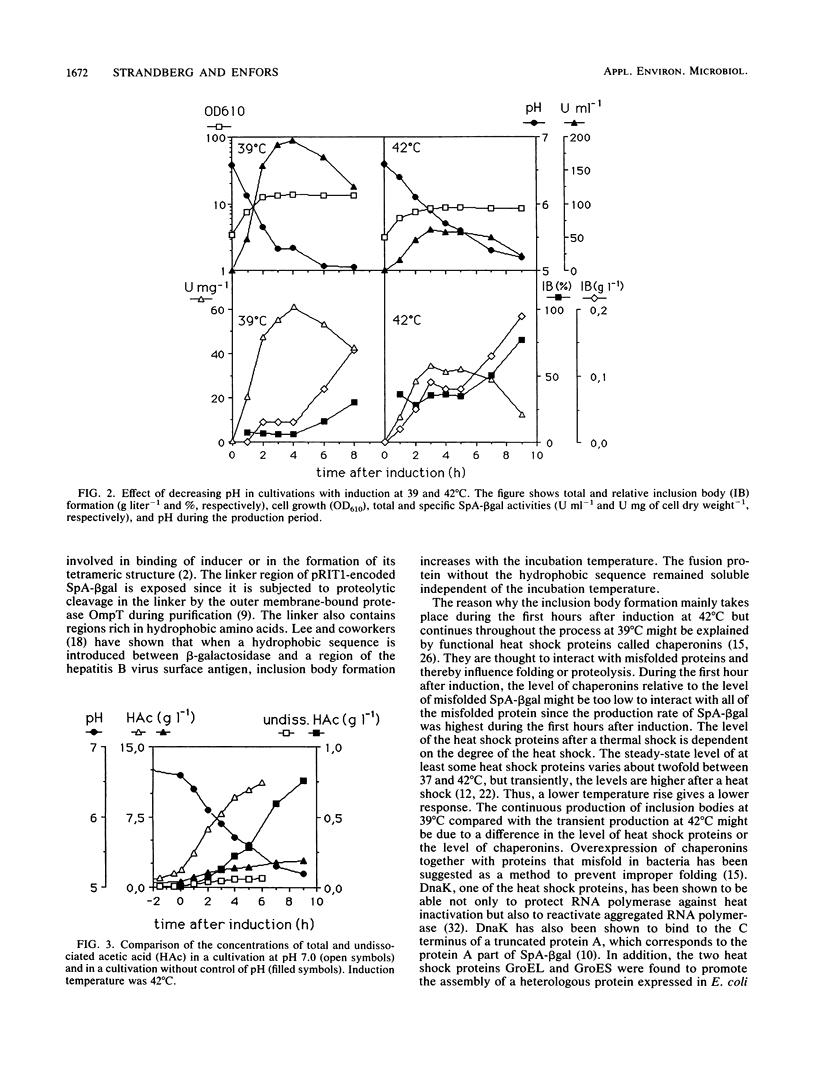
Abstract
Different parameters that influenced the formation of inclusion bodies in Escherichia coli during production of a fused protein consisting of protein A from Staphylococcus aureus and beta-galactosidase from E. coli were examined. The intracellular expression of the fused protein was controlled by the pR promoter and its temperature-sensitive repressor. The induction temperature, the pH of the cultivation medium, and changes in the amino acid sequence in the linker region between protein A and beta-galactosidase had a profound effect on the formation of inclusion bodies. At 42 degrees C, inclusion bodies were formed only during the first hours after induction, and thereafter all the recombinant protein that was further produced appeared in a soluble and active state. Production at 39 and 44 degrees C resulted in inclusion body formation throughout the production period with 15 to 20% of the produced recombinant protein appearing as inclusion bodies. Cultivating cells without control of pH caused inclusion body formation throughout the induction period, and inclusion body formation increased with decreasing pH, and at least part of the insoluble protein was formed from the pool of soluble fusion protein within the cell. Changes in the amino acid sequence in the linker region between the two parts of the fusion protein abolished inclusion body formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishai W. R., Rappuoli R., Murphy J. R. High-level expression of a proteolytically sensitive diphtheria toxin fragment in Escherichia coli. J Bacteriol. 1987 Nov;169(11):5140–5151. doi: 10.1128/jb.169.11.5140-5151.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J. J., Kim E., Telford J. N., Wong E. Y., Tacon W. C., Shuler M. L., Wilson D. B. Effects of temperature on Escherichia coli overproducing beta-lactamase or human epidermal growth factor. Appl Environ Microbiol. 1990 Jan;56(1):104–111. doi: 10.1128/aem.56.1.104-111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington C. A., Hinton M., Chopra I. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J Appl Bacteriol. 1990 Jan;68(1):69–74. doi: 10.1111/j.1365-2672.1990.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hellebust H., Uhlén M., Enfors S. O. Interaction between heat shock protein DnaK and recombinant staphylococcal protein A. J Bacteriol. 1990 Sep;172(9):5030–5034. doi: 10.1128/jb.172.9.5030-5034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. W., Hirshfield I. N. Low-pH-induced effects on patterns of protein synthesis and on internal pH in Escherichia coli and Salmonella typhimurium. Appl Environ Microbiol. 1990 Apr;56(4):1038–1045. doi: 10.1128/aem.56.4.1038-1045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Neupert W., Hartl F. U. Protein-catalysed protein folding. Trends Biotechnol. 1990 May;8(5):126–131. doi: 10.1016/0167-7799(90)90153-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Choi Y. C., Yu M. H. Effect of the N-terminal hydrophobic sequence of hepatitis B virus surface antigen on the folding and assembly of hybrid beta-galactosidase in Escherichia coli. Eur J Biochem. 1990 Jan 26;187(2):417–424. doi: 10.1111/j.1432-1033.1990.tb15320.x. [DOI] [PubMed] [Google Scholar]
- Luli G. W., Strohl W. R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol. 1990 Apr;56(4):1004–1011. doi: 10.1128/aem.56.4.1004-1011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L. Fusions to staphylococcal protein A. Methods Enzymol. 1990;185:144–161. doi: 10.1016/0076-6879(90)85015-g. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Rüther U. pUR 250 allows rapid chemical sequencing of both DNA strands of its inserts. Nucleic Acids Res. 1982 Oct 11;10(19):5765–5772. doi: 10.1093/nar/10.19.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond C. V., Kroll R. G., Booth I. R. The effect of food preservatives on pH homeostasis in Escherichia coli. J Gen Microbiol. 1984 Nov;130(11):2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]


