Abstract
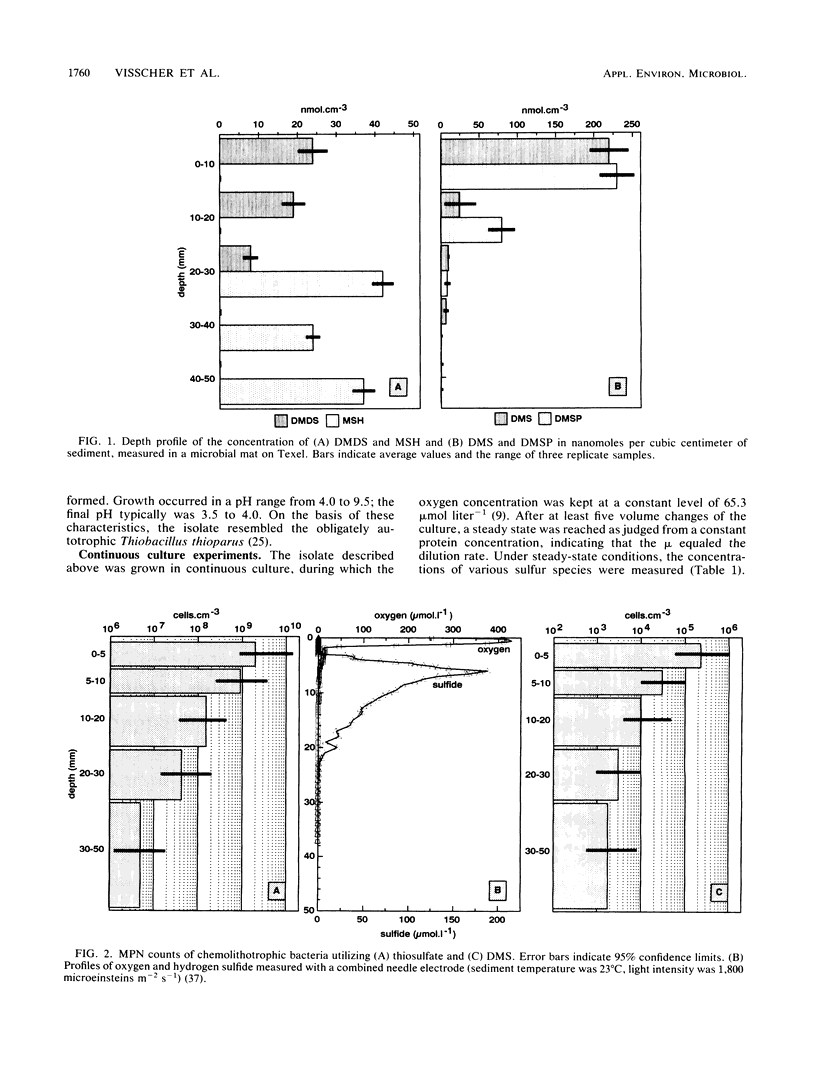
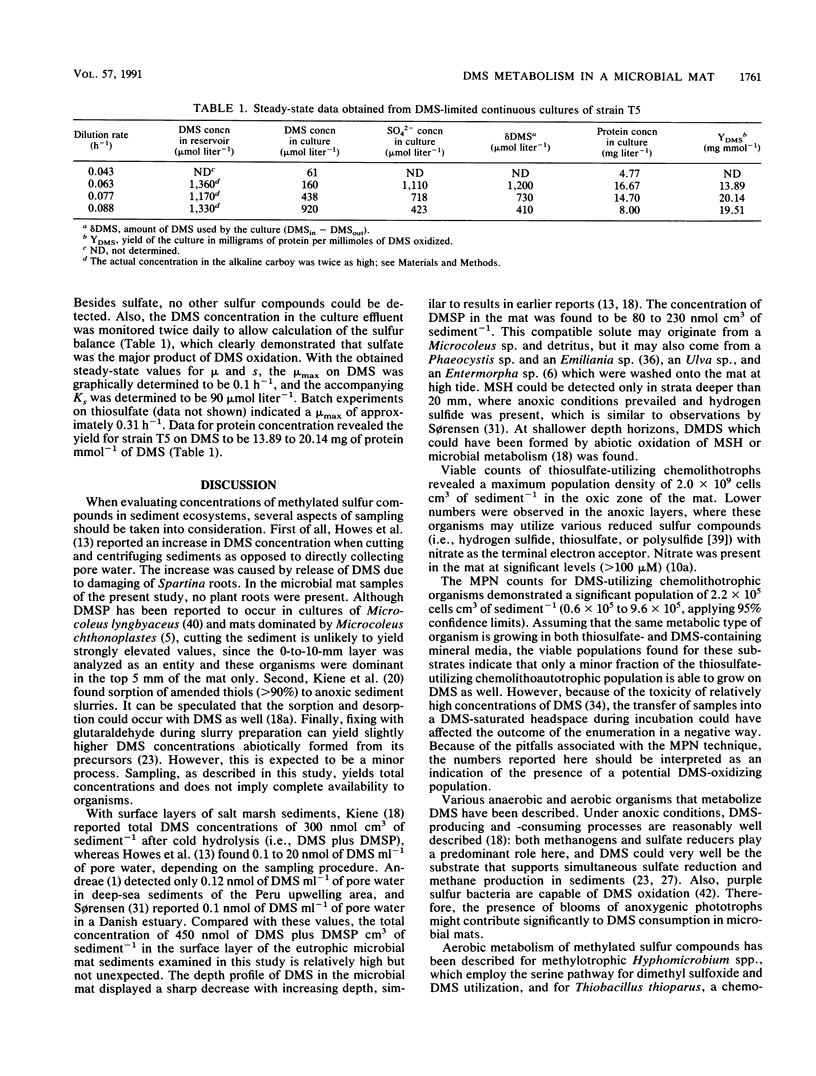
The concentrations of the volatile organic sulfur compounds methanethiol, dimethyl disulfide, and dimethyl sulfide (DMS) and the viable population capable of DMS utilization in laminated microbial ecosystems were evaluated. Significant levels of DMS and dimethyl disulfide (maximum concentrations of 220 and 24 nmol cm3 of sediment-1, respectively) could be detected only at the top 20 mm of the microbial mat, whereas methanethiol was found only at depth horizons from 20 to 50 mm (maximum concentration of 42 nmol cm3 of sediment-1). DMS concentrations in the surface layer doubled after cold hydrolysis of its precursor, dimethylsulfoniopropionate. Most-probable-number counts revealed 2.2 x 10(5) cells cm3 of sediment-1, in the 0- to 5-mm depth horizon, capable of growth on DMS as the sole source of energy. An obligately chemolithoautotrophic bacillus designated strain T5 was isolated from the top layer of the marine sediment. Continuous culture studies in which DMS was the growth-limiting substrate revealed a maximum specific growth rate of 0.10 h-1 and a saturation constant of 90 mumol liter-1 for aerobic growth on this substrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHALLENGER F., BYWOOD R., THOMAS P., HAYWARD B. J. Studies on biological methylation. XVII. The natural occurrence and chemical reactions of some thetins. Arch Biochem Biophys. 1957 Jul;69:514–523. doi: 10.1016/0003-9861(57)90516-7. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa T., Mikami E. Removal of methanethiol, dimethyl sulfide, dimethyl disulfide, and hydrogen sulfide from contaminated air by Thiobacillus thioparus TK-m. Appl Environ Microbiol. 1989 Mar;55(3):555–558. doi: 10.1128/aem.55.3.555-558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Biochemistry of the chemolithotrophic oxidation of inorganic sulphur. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):499–528. doi: 10.1098/rstb.1982.0094. [DOI] [PubMed] [Google Scholar]
- Kiene R. P., Malloy K. D., Taylor B. F. Sulfur-containing amino acids as precursors of thiols in anoxic coastal sediments. Appl Environ Microbiol. 1990 Jan;56(1):156–161. doi: 10.1128/aem.56.1.156-161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Taylor B. F. Demethylation of dimethylsulfoniopropionate and production of thiols in anoxic marine sediments. Appl Environ Microbiol. 1988 Sep;54(9):2208–2212. doi: 10.1128/aem.54.9.2208-2212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Visscher P. T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987 Oct;53(10):2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Suylen G. M., Kuenen J. G. Chemostat enrichment and isolation of Hyphomicrobium EG. A dimethyl-sulphide oxidizing methylotroph and reevaluation of Thiobacillus MS1. Antonie Van Leeuwenhoek. 1986;52(4):281–293. doi: 10.1007/BF00428640. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Zeyer J., Eicher P., Wakeham S. G., Schwarzenbach R. P. Oxidation of dimethyl sulfide to dimethyl sulfoxide by phototrophic purple bacteria. Appl Environ Microbiol. 1987 Sep;53(9):2026–2032. doi: 10.1128/aem.53.9.2026-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Doemel W. N., Brock T. D. Production of volatile sulfur compounds during the decomposition of algal mats. Appl Environ Microbiol. 1977 Dec;34(6):859–860. doi: 10.1128/aem.34.6.859-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


