Abstract
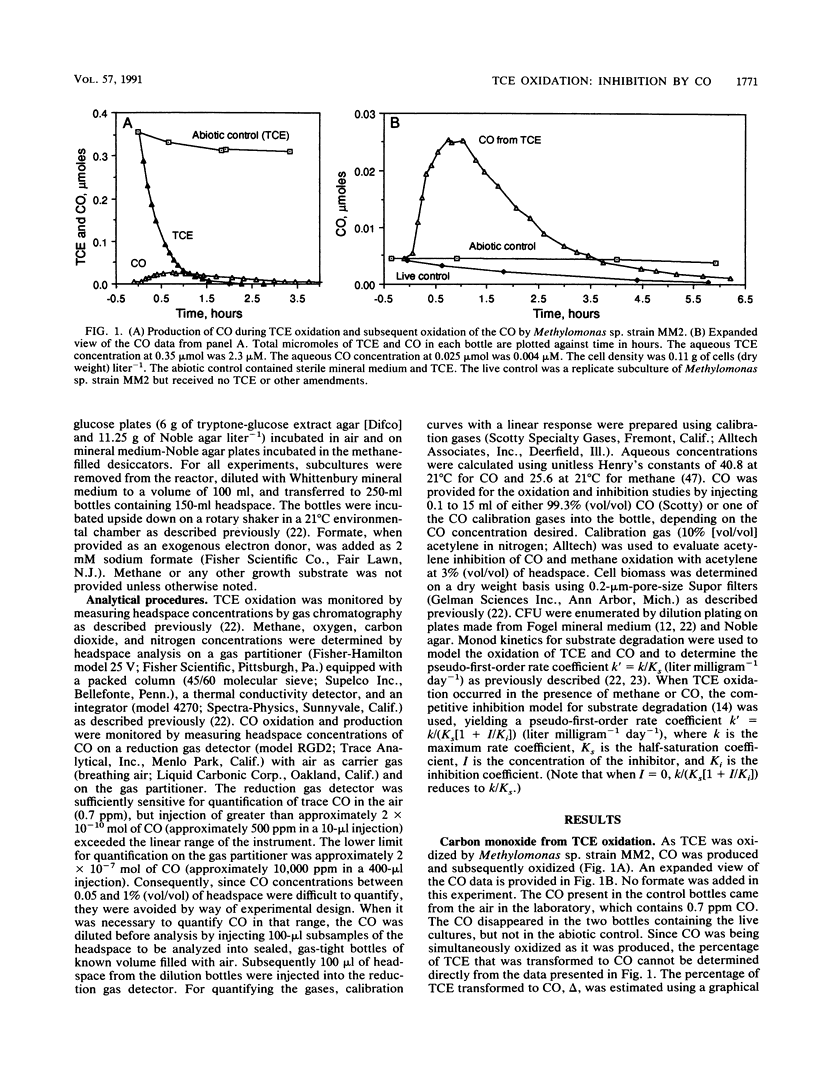
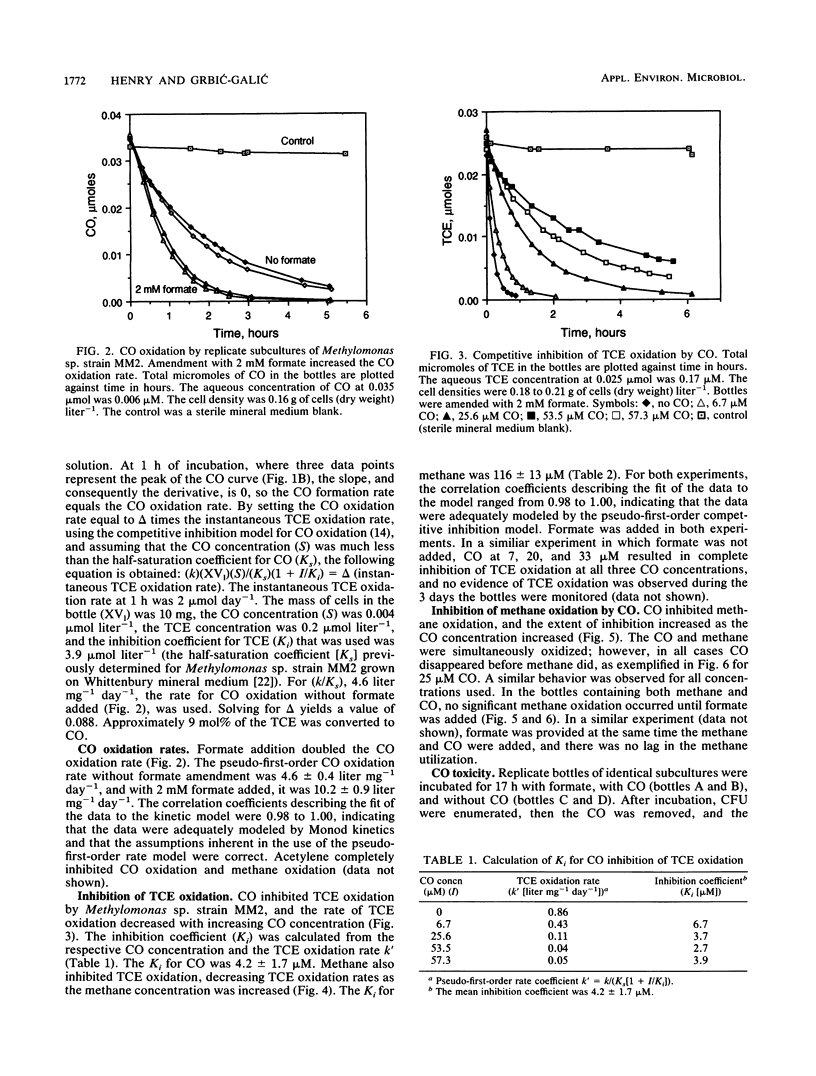
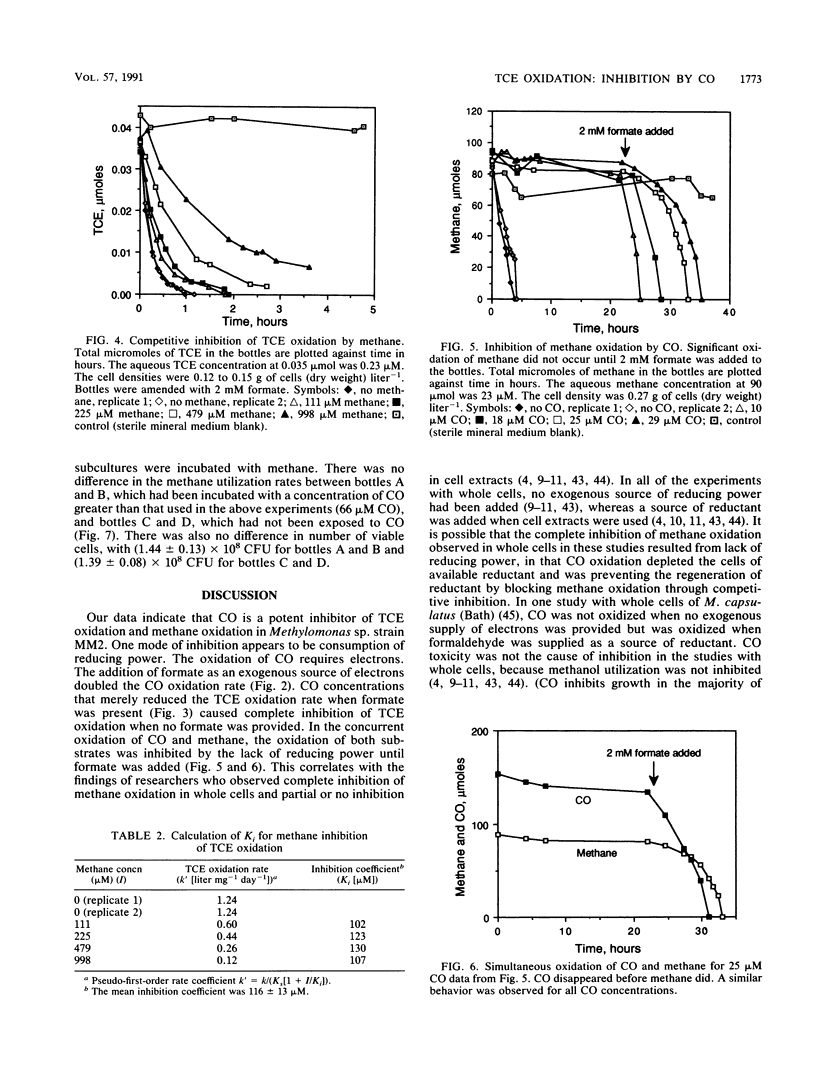
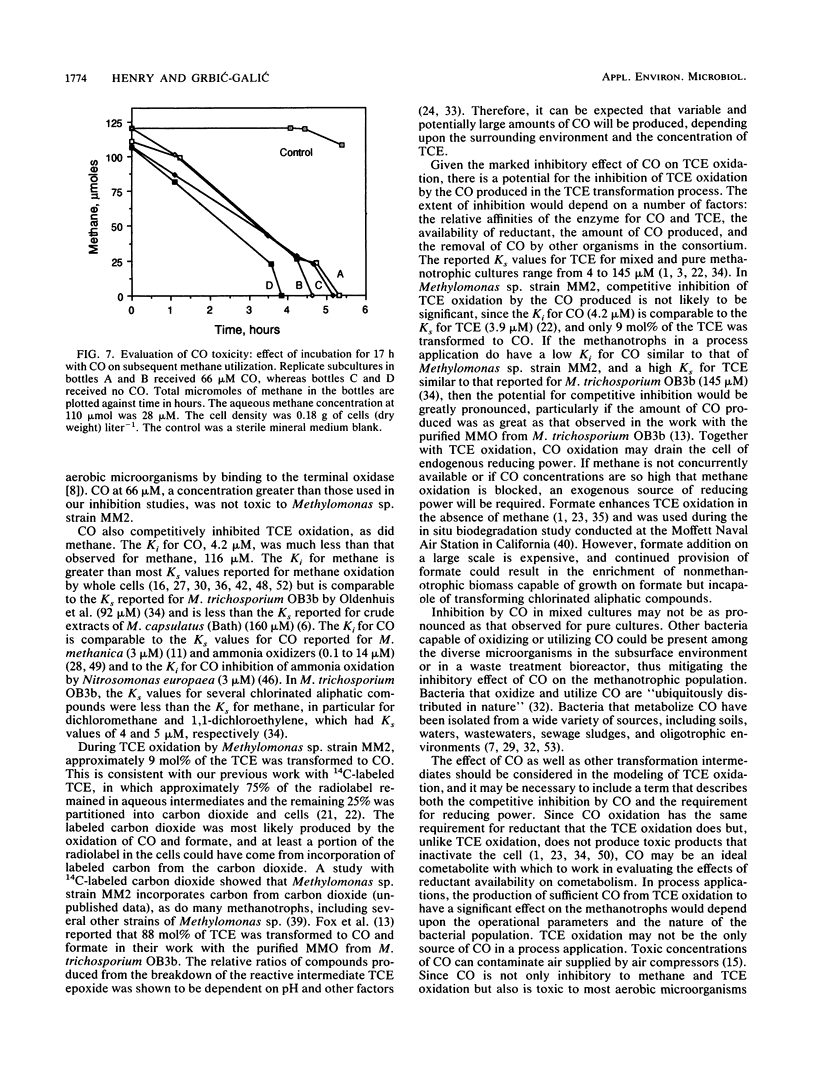
Inhibition of trichloroethylene (TCE) oxidation by the transformation intermediate carbon monoxide (CO) was evaluated with the aquifer methanotroph Methylomonas sp. strain MM2. CO was a TCE transformation intermediate. During TCE oxidation, approximately 9 mol% of the TCE was transformed to CO. CO was oxidized by Methylomonas sp. strain MM2, and when formate was provided as an electron donor, the CO oxidation rate doubled. The rate of CO oxidation without formate was 4.6 liter mg (dry weight)-1 day-1, and the rate with formate was 10.2 liter mg (dry weight)-1 day-1. CO inhibited TCE oxidation, both by exerting a demand for reductant and through competitive inhibition. The Ki for CO inhibition of TCE oxidation, 4.2 microM, was much less than the Ki for methane inhibition of TCE oxidation, 116 microM. CO also inhibited methane oxidation, and the degree of inhibition increased with increasing CO concentration. When CO was present, formate amendment was necessary for methane oxidation to occur and both substrates were simultaneously oxidized. CO at a concentration greater than that used in the inhibition studies was not toxic to Methylomonas sp. strain MM2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Cohen L., McCarty P. L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991 Jan;57(1):228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusseau G. A., Tsien H. C., Hanson R. S., Wackett L. P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation. 1990;1(1):19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Some properties of a soluble methane mono-oxygenase from Methylococcus capsulatus strain Bath. Biochem J. 1976 Aug 1;157(2):495–497. doi: 10.1042/bj1570495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H., Whittenbury R. An improved assay for bacterial methane mono-oxygenase: some properties of the enzyme from Methylomonas methanica. Biochem J. 1975 Nov;151(2):459–462. doi: 10.1042/bj1510459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Mitton J. R. Cytochromes of two methane-utilizing bacteria. FEBS Lett. 1973 Dec 1;37(2):335–338. doi: 10.1016/0014-5793(73)80491-0. [DOI] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Fogel M. M., Taddeo A. R., Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986 Apr;51(4):720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. G., Borneman J. G., Wackett L. P., Lipscomb J. D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990 Jul 10;29(27):6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- Harrison D. E. Studies on the affinity of methanol--and methane--utilizing bacteria for their carbon substrates. J Appl Bacteriol. 1973 Jun;36(2):301–308. doi: 10.1111/j.1365-2672.1973.tb04106.x. [DOI] [PubMed] [Google Scholar]
- Henry S. M., Grbić-Galić D. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer. Appl Environ Microbiol. 1991 Jan;57(1):236–244. doi: 10.1128/aem.57.1.236-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschler D., Hoos W. R., Fetz H., Dallmeier E., Metzler M. Reactions of trichloroethylene epoxide in aqueous systems. Biochem Pharmacol. 1979;28(4):543–548. doi: 10.1016/0006-2952(79)90251-x. [DOI] [PubMed] [Google Scholar]
- Hubley J. H., Mitton J. R., Wilkinson J. F. The oxidation of carbon monoxide by methane-oxidizing bacteria. Arch Mikrobiol. 1974 Feb 13;95(4):365–368. doi: 10.1007/BF02451778. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Oxidation of carbon monoxide by bacteria. Int Rev Cytol. 1983;81:1–32. doi: 10.1016/s0074-7696(08)62333-5. [DOI] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Oxidation of trichloroethylene by liver microsomal cytochrome P-450: evidence for chlorine migration in a transition state not involving trichloroethylene oxide. Biochemistry. 1982 Mar 2;21(5):1090–1097. doi: 10.1021/bi00534a041. [DOI] [PubMed] [Google Scholar]
- Oldenhuis R., Oedzes J. Y., van der Waarde J. J., Janssen D. B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991 Jan;57(1):7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A. Microbial Oxidation of Hydrocarbons: Properties of a Soluble Methane Monooxygenase from a Facultative Methane-Utilizing Organism, Methylobacterium sp. Strain CRL-26. Appl Environ Microbiol. 1982 Nov;44(5):1130–1137. doi: 10.1128/aem.44.5.1130-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovskaia V. A., Liudvichenko E. S., Kryshtab T. P., Zhukov V. G., Sokolov I. G. Rol' ékzogennoi uglekisloty v metabolizme metanokisliaiushchikh bakterii. Mikrobiologiia. 1980 Sep-Oct;49(5):687–694. [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Effect of metal-binding and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch Microbiol. 1977 Jul 26;114(1):71–76. doi: 10.1007/BF00429633. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C., Dular U. Competitive inhibition of ammonia oxidation in Nitrosomonas europaea by methane, carbon monoxide or methanol. FEBS Lett. 1976 Dec 15;72(1):117–120. doi: 10.1016/0014-5793(76)80825-3. [DOI] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang D. C., Suzuki I. Cytochrome c554 as a possible electron donor in the hydroxylation of ammonia and carbon monoxide in Nitrosomonas europaea. Can J Biochem. 1982 Nov;60(11):1018–1024. doi: 10.1139/o82-131. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Householder S. R. Toxicity of Trichloroethylene to Pseudomonas putida F1 Is Mediated by Toluene Dioxygenase. Appl Environ Microbiol. 1989 Oct;55(10):2723–2725. doi: 10.1128/aem.55.10.2723-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- Wilkinson T. G., Harrison D. E. The affinity for methane and methanol of mixed cultures grown on methane in continuous culture. J Appl Bacteriol. 1973 Jun;36(2):309–313. doi: 10.1111/j.1365-2672.1973.tb04107.x. [DOI] [PubMed] [Google Scholar]


