Abstract
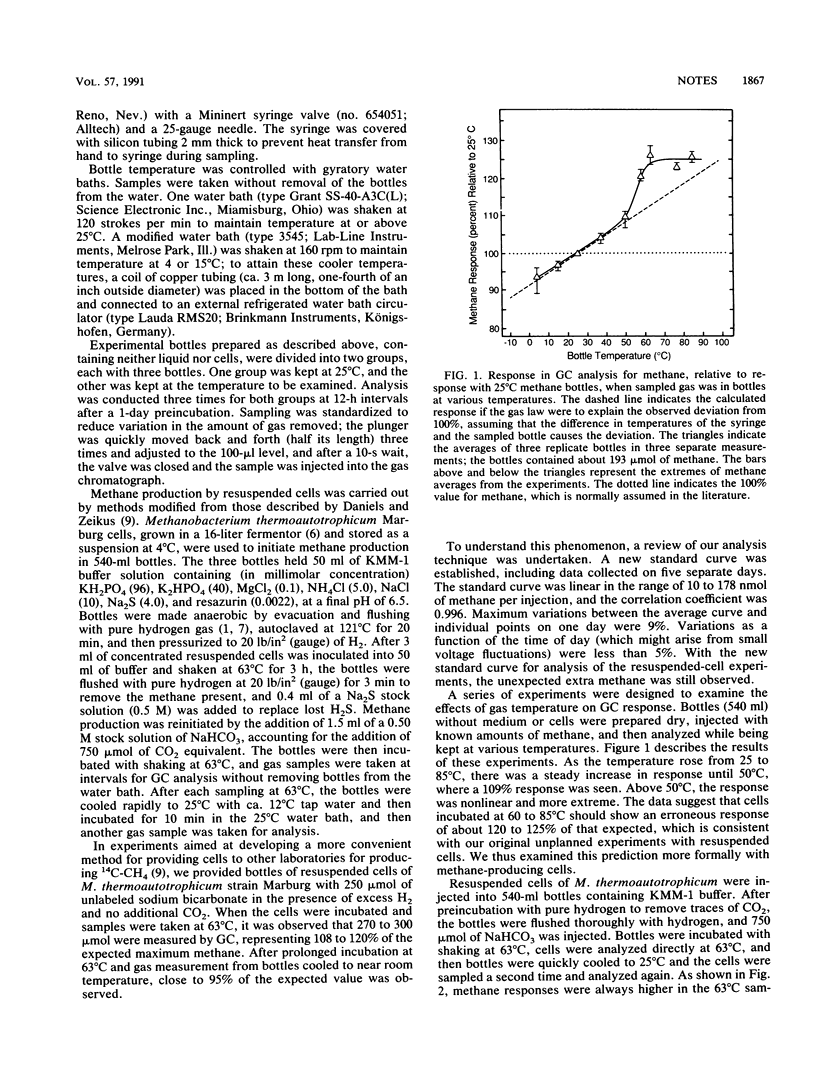
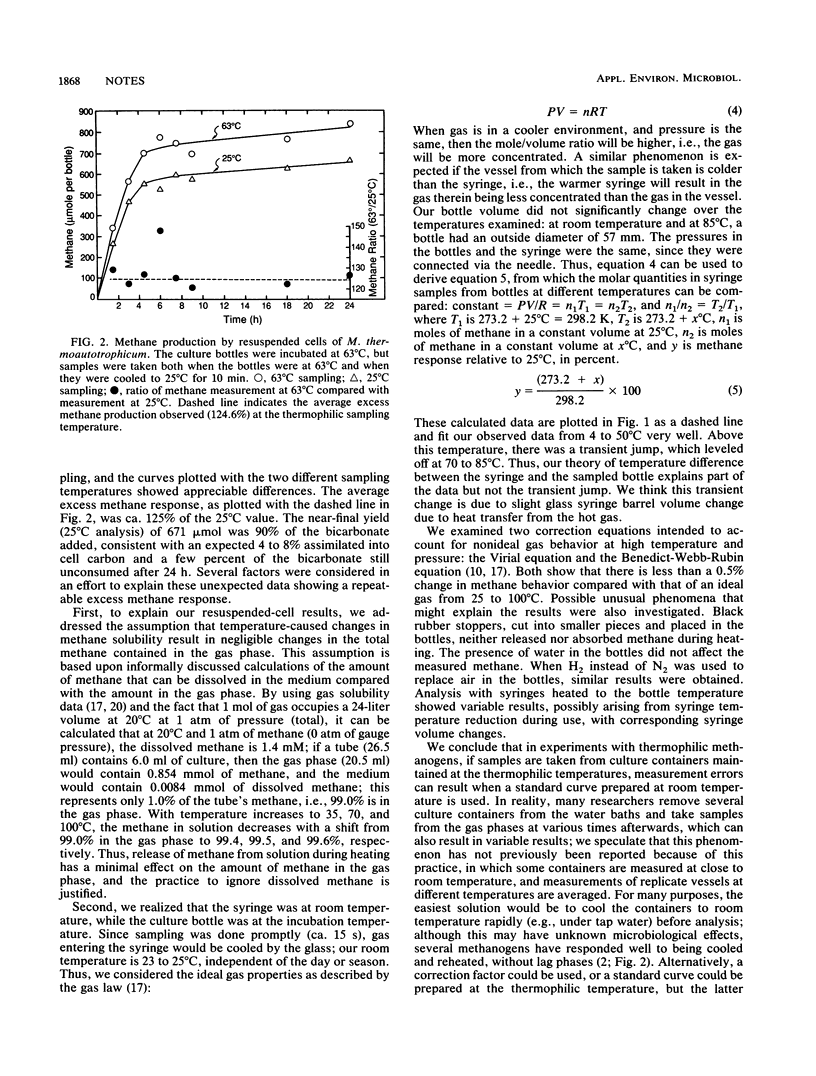
Unexpected errors in methane measurement by gas chromatography occurred when samples at thermophilic temperatures were analyzed. With a standard curve prepared at room temperature (25°C), stoppered bottles incubated and sampled at 37 to 85°C showed more methane upon analysis than bottles incubated at 25°C: values at 50, 63, and 85°C were 109, 126, and 125%, respectively, of the 25°C value. All variation between 4 and 50°C can be explained by the temperature difference between culture bottle and sampling syringe, and the variation of methane concentration can be predicted by the gas law. Between 50 and 63°C, there was a more dramatic rise than predicted by theory. These variations are important to consider if thermophilic methane production is to be measured accurately. Methods to avoid errors are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay N., Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987 Jul;53(7):1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay N., Sparling R., Daniels L. Relationship of formate to growth and methanogenesis by Methanococcus thermolithotrophicus. Appl Environ Microbiol. 1986 Nov;52(5):1080–1085. doi: 10.1128/aem.52.5.1080-1085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Belay N., Rajagopal B. S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol. 1986 Apr;51(4):703–709. doi: 10.1128/aem.51.4.703-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


