Abstract
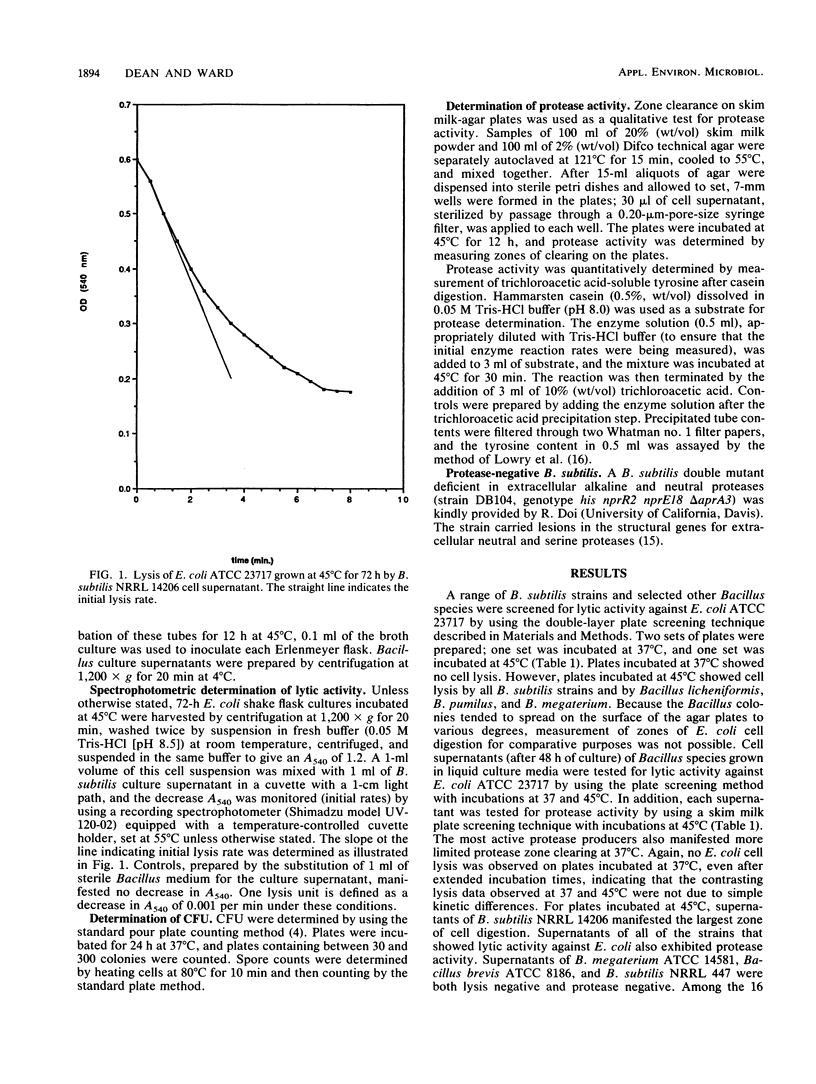
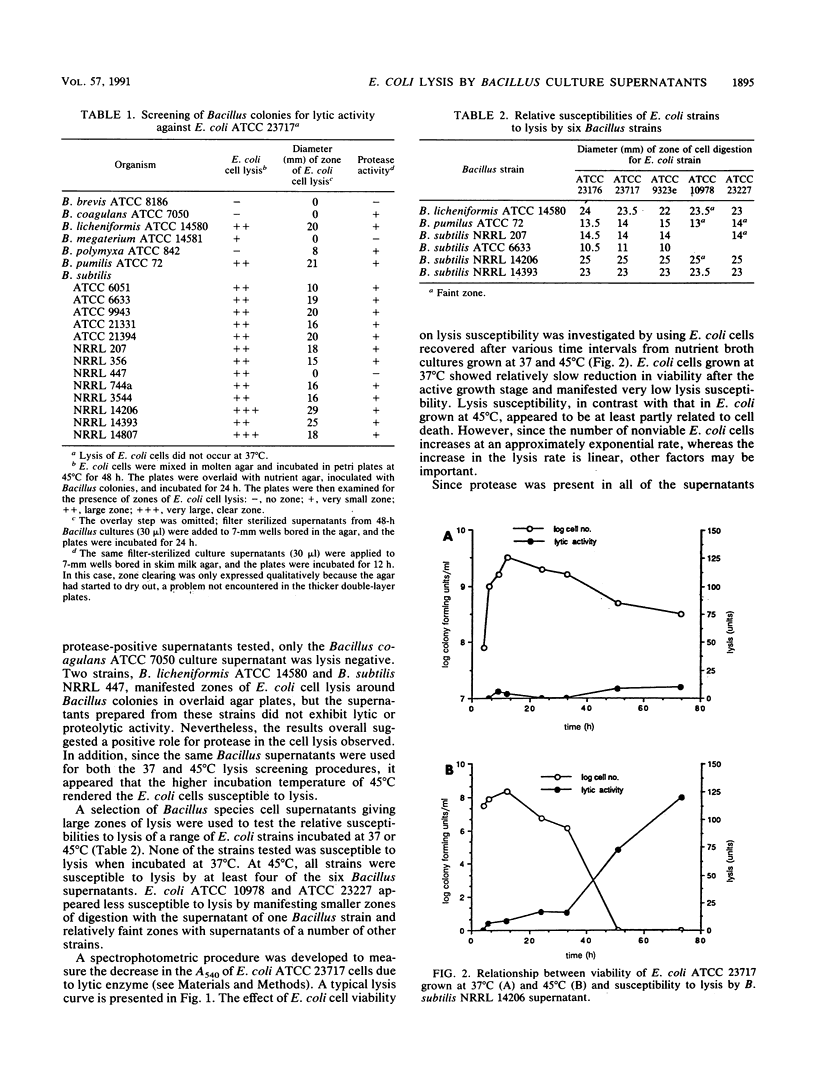
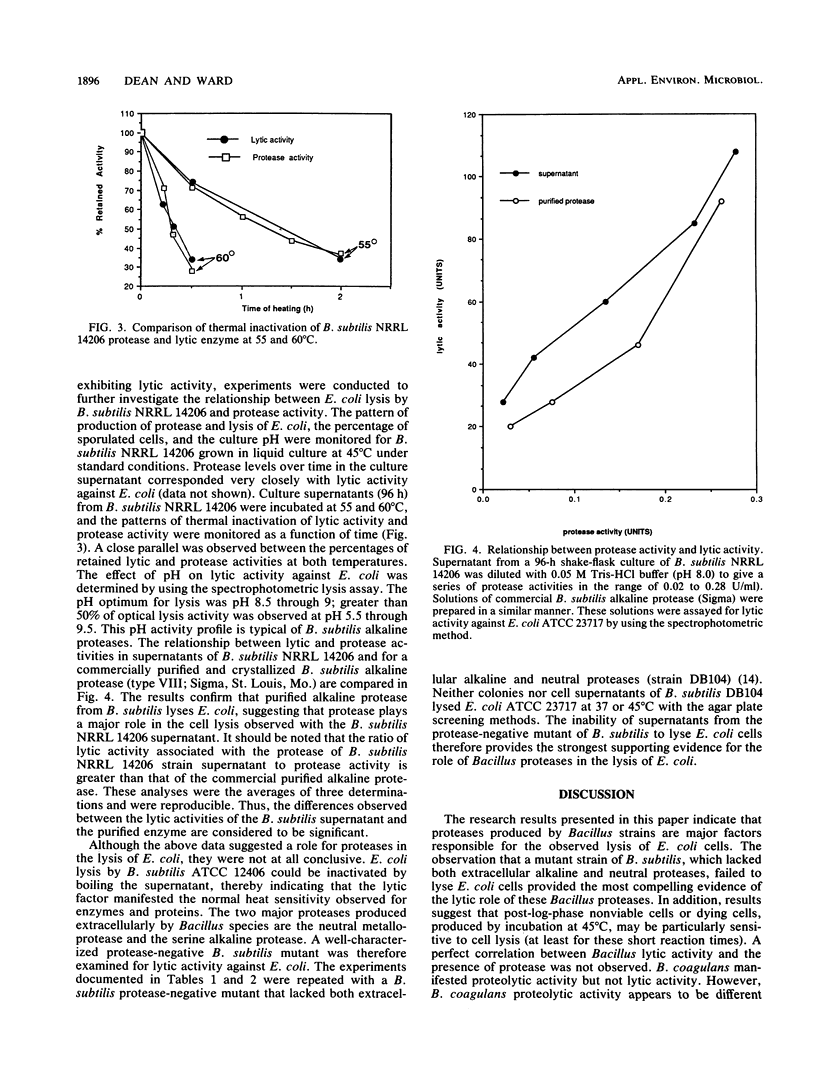
Escherichia coli cells were found to be sensitive to lysis by the supernatants of a variety of protease-positive Bacillus species when treated at 45 degrees C but not when treated at 37 degrees C. Different E. coli strains manifested different lysis sensitivities when treated at 45 degrees C. When the lysis rates of E. coli cells at various stages of growth were investigated, post-exponential-phase cells were shown to be most sensitive to lysis. The lysis rate was inversely related to cell viability, and susceptibility appeared to be at least partly due to lysis of dead E. coli cells. A close relation was observed between levels of lysis activity and proteolytic activity. A Bacillus subtilis mutant lacking alkaline and neutral protease activity failed to lyse E. coli cells. It was concluded that Bacillus proteases played a major role in the observed E. coli lysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker T., Ogez J. R., Builder S. E. Downstream processing of proteins. Biotechnol Adv. 1983;1(2):247–261. doi: 10.1016/0734-9750(83)90591-8. [DOI] [PubMed] [Google Scholar]
- Desai A. J., Dhala S. A. Purification and properties of proteolytic enzymes from thermophilic actinomycetes. J Bacteriol. 1969 Oct;100(1):149–155. doi: 10.1128/jb.100.1.149-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchener B. J., Egan A. F. Outer-membrane damage in sublethally heated Escherichia coli K-12. Can J Microbiol. 1977 Mar;23(3):311–318. doi: 10.1139/m77-046. [DOI] [PubMed] [Google Scholar]
- Ismaeel N., Furr J. R., Russell A. D. Reversal of the surface effects of chlorhexidine diacetate on cells of Providencia stuartii. J Appl Bacteriol. 1986 Nov;61(5):373–381. doi: 10.1111/j.1365-2672.1986.tb04299.x. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984 Oct;160(1):442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Musílková M., Fencl Z., Seichertová O. Release of Aspergillus niger Protoplasts by Penicillium purpurogenum enzymes. Folia Microbiol (Praha) 1969;14(1):47–50. doi: 10.1007/BF02869398. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Peptidoglycans (mucopeptides): structure, function, and variations. Ann N Y Acad Sci. 1974 May 10;235(0):29–51. doi: 10.1111/j.1749-6632.1974.tb43255.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Storm D. R. Disruption of the Escherichia coli outer membrane permeability barrier by immobilized polymyxin B. J Antibiot (Tokyo) 1977 Dec;30(12):1087–1092. doi: 10.7164/antibiotics.30.1087. [DOI] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F., Jr Lysis of Escherichia coli by ethylenediaminetetraacetate and phospholipases as measured by beta-galactosidase activity. J Bacteriol. 1967 Oct;94(4):934–941. doi: 10.1128/jb.94.4.934-941.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchido T., Katsui N., Takeuchi A., Takano M., Shibasaki I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl Environ Microbiol. 1985 Aug;50(2):298–303. doi: 10.1128/aem.50.2.298-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D., Marcus D. Disintegration of microorganisms. Adv Biotechnol Processes. 1988;8:51–96. [PubMed] [Google Scholar]


