Abstract
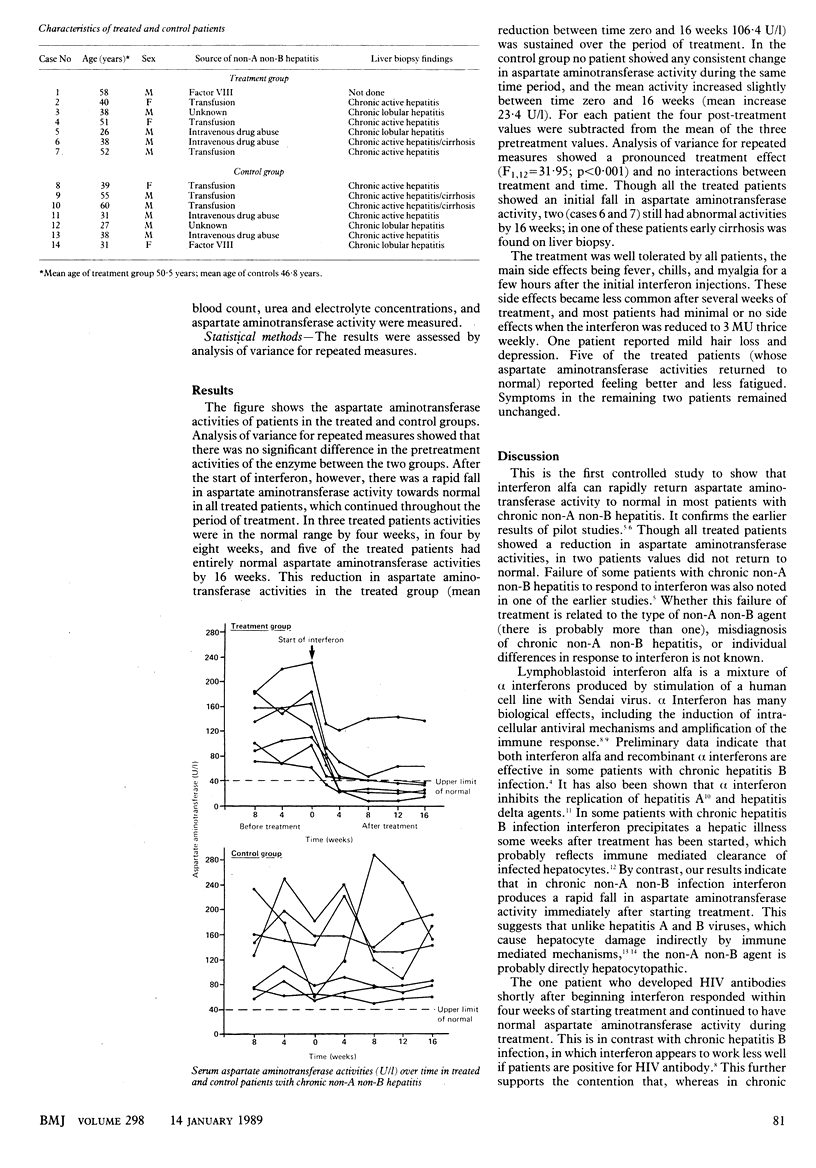
OBJECTIVE--To determine the effect of low dose interferon alfa (human lymphoblastoid interferon) on aminotransferase activities in chronic non-A non-B hepatitis. DESIGN--Prospective randomised controlled parallel group study of active treatment versus no treatment carried out over 16 weeks and preceded by baseline measurements at weeks 8 and 4 and time zero. SETTING--HEPATOLOGY outpatient clinics in secondary referral centres. PATIENTS--Fourteen adults with histologically proved chronic hepatitis and persistently raised aminotransferase activities for six months or more. INTERVENTIONS--Seven patients randomised to receive interferon alfa 5 megaunits (MU) daily for one week, reducing to 5 MU thrice weekly for seven weeks, then 3 MU thrice weekly for eight weeks. Controls not treated. END POINT--Control of hepatic enzyme activity in chronic non-A non-B hepatitis. MEASUREMENTS AND MAIN RESULTS--Serum aspartate aminotransferase activity remained raised in controls (mean increase in study period 23.4 U/l) but fell rapidly to normal in the treated group (mean decrease 106.4 U/l). In four cases values were normal by eight weeks and in five cases by 16 weeks. Only minor side effects were recorded (fever, myalgia), which became less common as treatment progressed. CONCLUSIONS--Continuous low dose interferon alfa reduces aspartate aminotransferase activity to normal in most patients with chronic non-A non-B hepatitis and may prevent progression to cirrhosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dienstag J. L. Non-A, non-B hepatitis. I. Recognition, epidemiology, and clinical features. Gastroenterology. 1983 Aug;85(2):439–462. [PubMed] [Google Scholar]
- Hoofnagle J. H., Mullen K. D., Jones D. B., Rustgi V., Di Bisceglie A., Peters M., Waggoner J. G., Park Y., Jones E. A. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986 Dec 18;315(25):1575–1578. doi: 10.1056/NEJM198612183152503. [DOI] [PubMed] [Google Scholar]
- Leggett B., Collins R., Prentice R., Powell L. W. CAH or SLE? Hepatology. 1986 Mar-Apr;6(2):341–342. doi: 10.1002/hep.1840060241. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Caruso L., Karayiannis P., Scully L. J., Harris J. R., Forster G. E., Thomas H. C. Diminished responsiveness of male homosexual chronic hepatitis B virus carriers with HTLV-III antibodies to recombinant alpha-interferon. Hepatology. 1987 Jul-Aug;7(4):719–723. doi: 10.1002/hep.1840070417. [DOI] [PubMed] [Google Scholar]
- Pappas S. C., Hoofnagle J. H., Young N., Straus S. E., Jones E. A. Treatment of chronic non-A, non-B hepatitis wih acyclovir: pilot study. J Med Virol. 1985 Jan;15(1):1–9. doi: 10.1002/jmv.1890150102. [DOI] [PubMed] [Google Scholar]
- Realdi G., Alberti A., Rugge M., Rigoli A. M., Tremolada F., Schivazappa L., Ruol A. Long-term follow-up of acute and chronic non-A, non-B post-transfusion hepatitis: evidence of progression to liver cirrhosis. Gut. 1982 Apr;23(4):270–275. doi: 10.1136/gut.23.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B. J., Doran M., Lever A. M., Webster A. D. Alpha-interferon therapy for non-A, non-B hepatitis transmitted by gammaglobulin replacement therapy. Lancet. 1987 Mar 7;1(8532):539–541. doi: 10.1016/s0140-6736(87)90178-4. [DOI] [PubMed] [Google Scholar]
- Vallbracht A., Gabriel P., Maier K., Hartmann F., Steinhardt H. J., Müller C., Wolf A., Manncke K. H., Flehmig B. Cell-mediated cytotoxicity in hepatitis A virus infection. Hepatology. 1986 Nov-Dec;6(6):1308–1314. doi: 10.1002/hep.1840060614. [DOI] [PubMed] [Google Scholar]


