Abstract
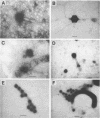
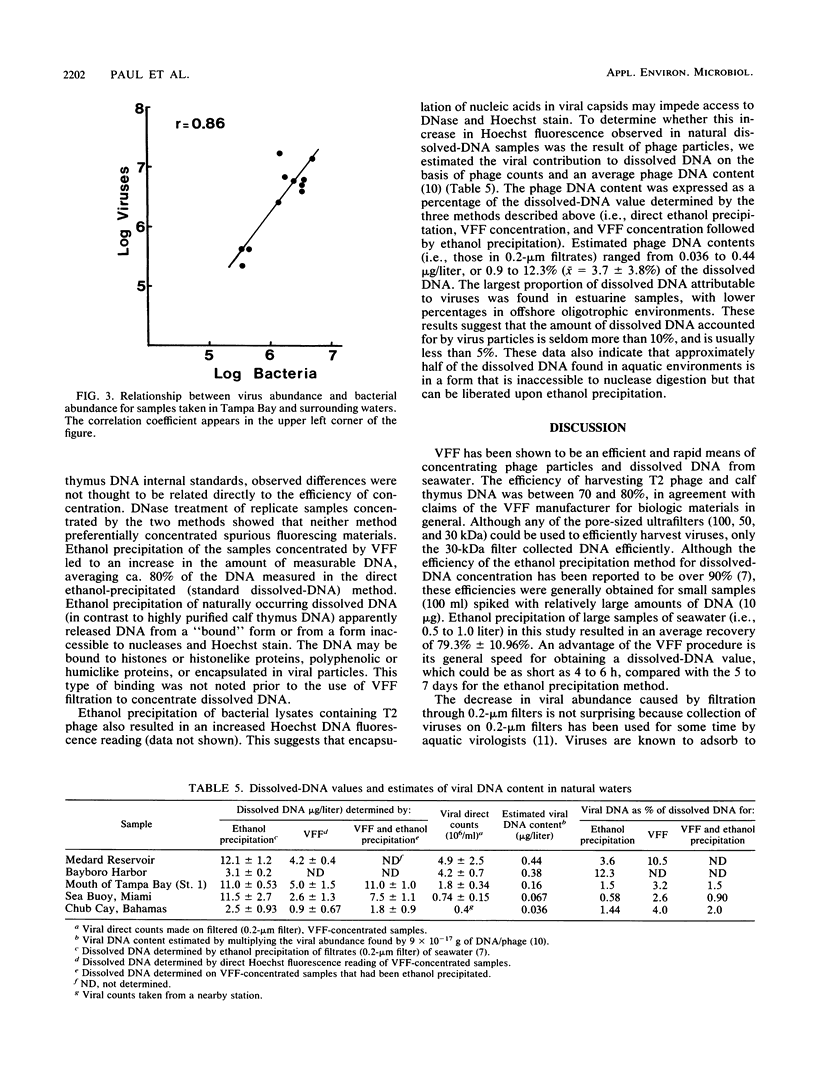
Vortex flow filtration (VFF) was used to concentrate viruses and dissolved DNA from freshwater and seawater samples taken in Florida, the Gulf of Mexico, and the Bahamas Bank. Recoveries of T2 phage and calf thymus DNA added to artificial seawater and concentrated by VFF were 72.8 and 80%, respectively. Virus concentrations determined by transmission electron microscopy of VFF-concentrated samples ranged from 3.4 x 10(7)/ml for a eutrophic Tampa Bay sample to 2.4 x 10(5) for an oligotrophic oceanic surface sample from the southeastern Gulf of Mexico. Viruslike particles were also observed in a sample taken from a depth of 1,500 m in the subtropical North Atlantic Ocean. Filtration of samples through Nuclepore or Durapore filters (pore size, 0.2 micron) prior to VFF reduced phage counts by an average of two-thirds. Measurement of dissolved-DNA content by Hoechst 33258 fluorescence in environmental samples concentrated by VFF yielded values only ca. 35% of those obtained for samples concentrated by ethanol precipitation (the standard dissolved-DNA method). However, ethanol precipitation of VFF-concentrated extracts resulted in an increase in measurable DNA, reaching 80% of the value obtained by the standard method. These results indicate that a portion of the naturally occurring dissolved DNA is in a form inaccessible to nucleases and Hoechst stain, perhaps bound to protein or other polymeric material, and is released upon ethanol precipitation. Viral DNA contents estimated from viral counts averaged only 3.7% (range, 0.9 to 12.3%) of the total dissolved DNA for samples from freshwater, estuarine, and offshore oligotrophic environments.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
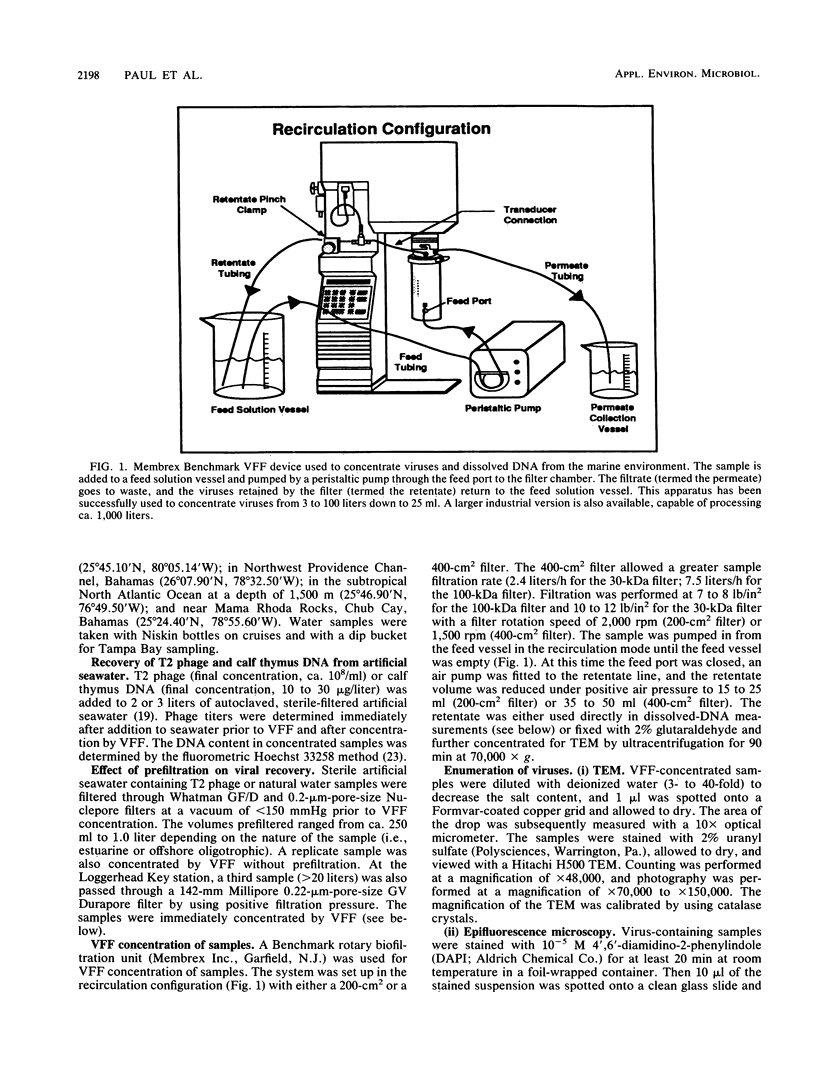
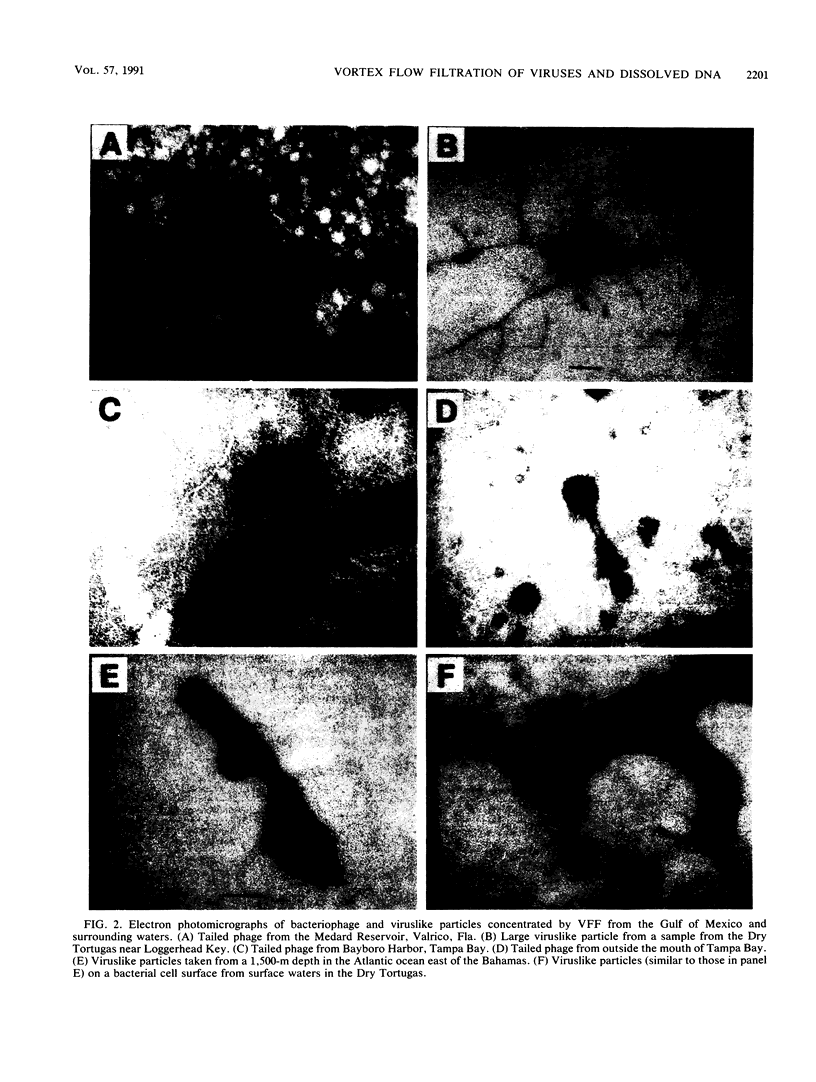


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baross J. A., Liston J., Morita R. Y. Ecological relationship between Vibrio parahaemolyticus and agar-digesting vibrios as evidenced by bacteriophage susceptibility patterns. Appl Environ Microbiol. 1978 Sep;36(3):500–505. doi: 10.1128/aem.36.3.500-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baross J. A., Liston J., Morita R. Y. Incidence of Vibrio parahaemolyticus bacteriophages and other Vibrio bacteriophages in marine samples. Appl Environ Microbiol. 1978 Sep;36(3):492–499. doi: 10.1128/aem.36.3.492-499.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børsheim K. Y., Bratbak G., Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990 Feb;56(2):352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. An evaluation of factors affecting the survival of Escherichia coli in sea water. IV. Bacteriophages. Appl Microbiol. 1960 Jul;8:254–256. doi: 10.1128/am.8.4.254-256.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflaun M. F., Paul J. H., Davis D. Simplified method for dissolved DNA determination in aquatic environments. Appl Environ Microbiol. 1986 Oct;52(4):654–659. doi: 10.1128/aem.52.4.654-659.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Cazares L., Thurmond J. Amplification of the rbcL gene from dissolved and particulate DNA from aquatic environments. Appl Environ Microbiol. 1990 Jun;56(6):1963–1966. doi: 10.1128/aem.56.6.1963-1966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jeffrey W. H., DeFlaun M. F. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987 Jan;53(1):170–179. doi: 10.1128/aem.53.1.170-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER R. A marine bacteriophage. Nature. 1955 Apr 16;175(4459):690–691. doi: 10.1038/175690a0. [DOI] [PubMed] [Google Scholar]
- Spencer R. INDIGENOUS MARINE BACTERIOPHAGES. J Bacteriol. 1960 Apr;79(4):614–614. doi: 10.1128/jb.79.4.614-614.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991 Mar;57(3):721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: ecological and taxonomical implications. Appl Environ Microbiol. 1979 Apr;37(4):774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]