Abstract
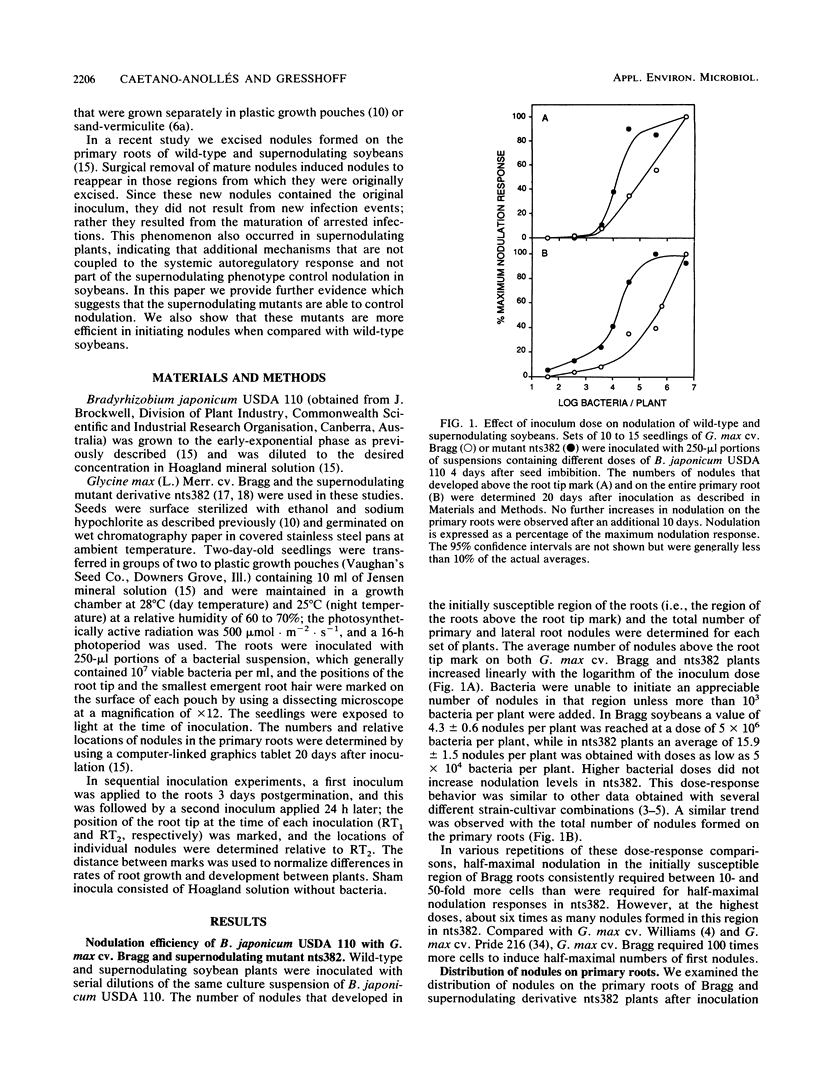
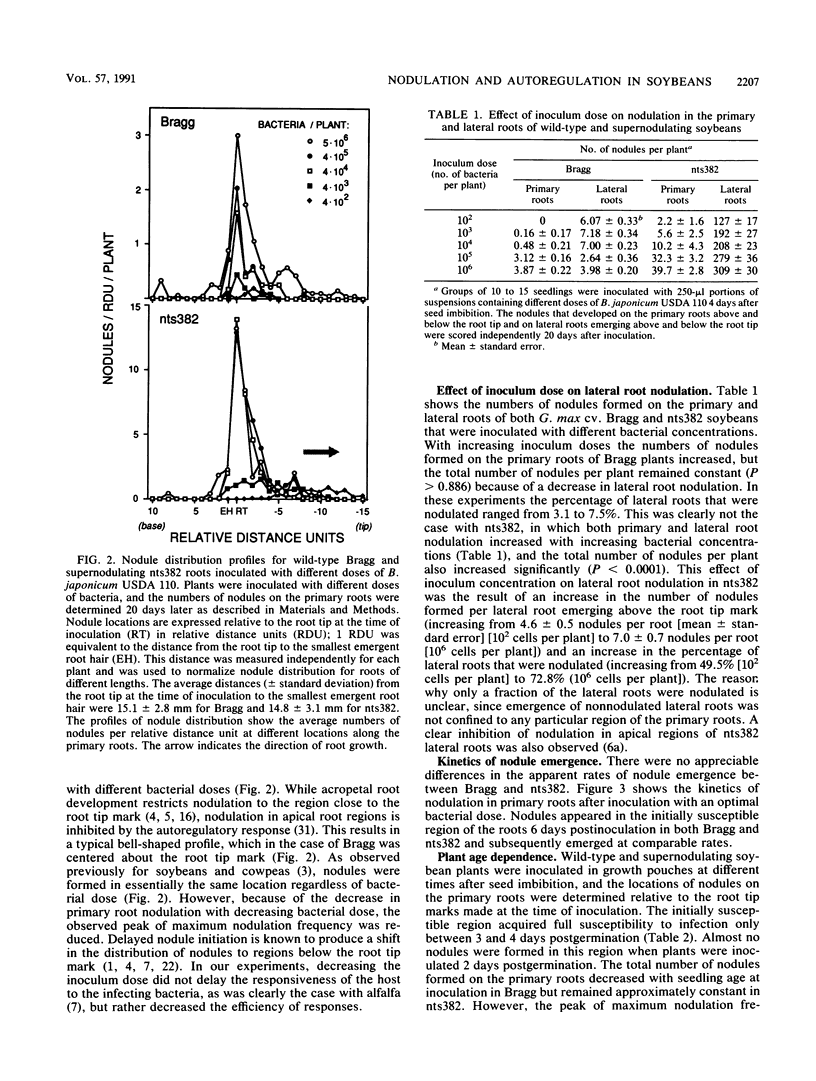
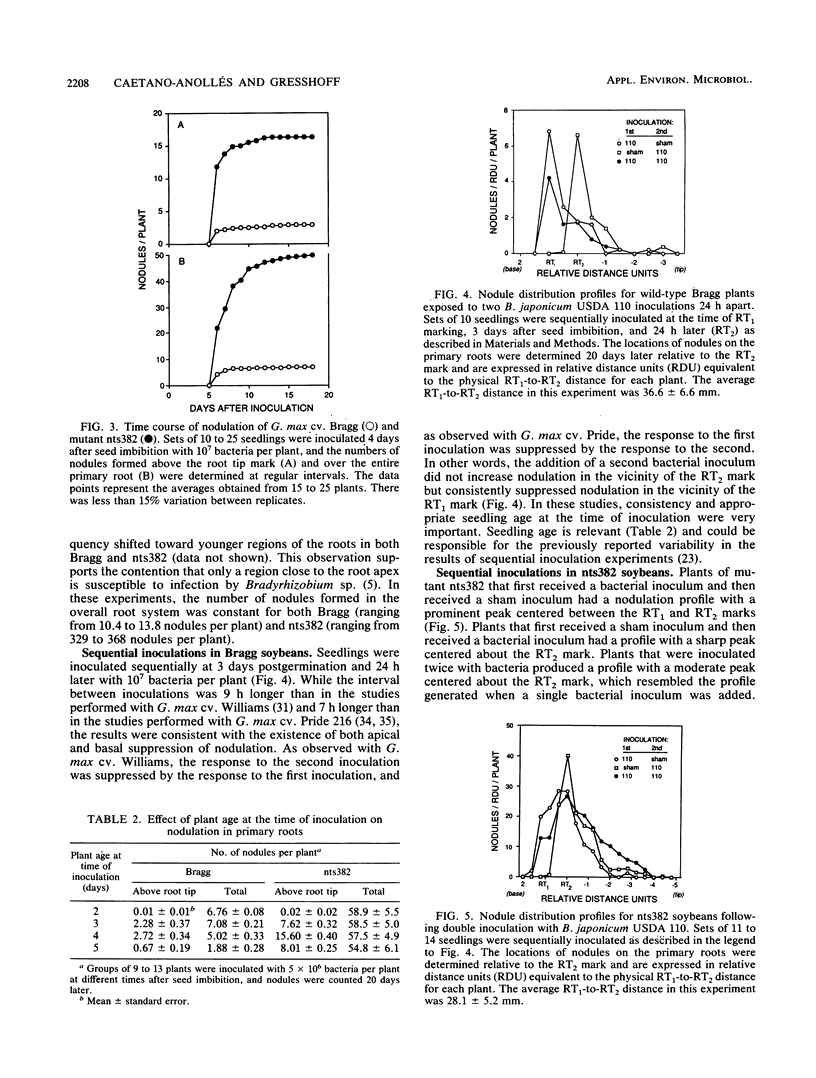
We compared the formation of nodules on the primary roots of a soybean cultivar (Glycine max (L.) Merr. cv. Bragg) and a supernodulating mutant derivative, nts382. Inoculation with Bradyrhizobium japonicum USDA 110 at different times after seed imbibition showed that the roots acquired full susceptibility to infection only between 3 and 4 days postgermination. When the plants were inoculated with serial dilutions of a bacterial suspension, the number of nodules formed in the initially susceptible region of the roots was linearly dependent on the logarithm of the inoculum dose until an optimum dose was reached. At least 10-fold-lower doses were required to induce half-maximal nodulation responses on nts382 than on the wild type. However, at optimal doses, about six times as many nodules formed in the initially susceptible region of the roots in nts382. Since there was no appreciable difference in the apparent rates of nodule emergence, the increased efficiency of nodule initiation in the supernodulating mutant could have resulted from a lower threshold of response to bacterial symbiotic signals. Two inoculations (24 h apart) of G. max cv. Bragg revealed that there was a host-mediated regulatory response that suppressed nodulation in younger portions of the primary roots, as reported previously for other soybean cultivar-Bradyrhizobium combinations. Similar experiments with nts382 revealed a comparable suppressive response, but this response was not as pronounced as it was in the wild type. This and other results suggest that there are additional control mechanisms for nodulation that are different from the systemic autoregulatory control of nodulation altered in supernodulating mutants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagwat A. A., Thomas J. Legume-Rhizobium interactions: cowpea root exudate elicits faster nodulation response by Rhizobium species. Appl Environ Microbiol. 1982 Apr;43(4):800–805. doi: 10.1128/aem.43.4.800-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Lesniak A. P., Bauer W. D. Efficiency of nodule initiation in cowpea and soybean. Plant Physiol. 1988 Apr;86(4):1210–1215. doi: 10.1104/pp.86.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Mills K. K., Crist D. K., Evans W. R., Bauer W. D. Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J Bacteriol. 1983 Jan;153(1):443–451. doi: 10.1128/jb.153.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Gresshoff P. M. Alfalfa Controls Nodulation during the Onset of Rhizobium-induced Cortical Cell Division. Plant Physiol. 1991 Feb;95(2):366–373. doi: 10.1104/pp.95.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Gresshoff P. M. Plant genetic control of nodulation. Annu Rev Microbiol. 1991;45:345–382. doi: 10.1146/annurev.mi.45.100191.002021. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Lagares A., Bauer W. D. Rhizobium meliloti exopolysaccharide Mutants Elicit Feedback Regulation of Nodule Formation in Alfalfa. Plant Physiol. 1990 Feb;92(2):368–374. doi: 10.1104/pp.92.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. J., McNeil D. L., Gresshoff P. M. A Supernodulation and Nitrate-Tolerant Symbiotic (nts) Soybean Mutant. Plant Physiol. 1985 May;78(1):34–40. doi: 10.1104/pp.78.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. J., McNeil D. L., Gresshoff P. M. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves A. C., Mathews A., Day D. A., Carter A. S., Carroll B. J., Gresshoff P. M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986 Oct;82(2):588–590. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremaud M. F., Harper J. E. Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol. 1989 Jan;89(1):169–173. doi: 10.1104/pp.89.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis : evidence for the involvement of lectin in nodulation. Plant Physiol. 1985 Mar;77(3):621–625. doi: 10.1104/pp.77.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron D. S., Pueppke S. G. Regulation of nodulation in the soybean-Rhizobium symbiosis : strain and cultivar variability. Plant Physiol. 1987 Aug;84(4):1391–1396. doi: 10.1104/pp.84.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984 May;75(1):125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J. E., Nakao P., Bohlool B. B., Gresshoff P. M. Lack of Systemic Suppression of Nodulation in Split Root Systems of Supernodulating Soybean (Glycine max [L.] Merr.) Mutants. Plant Physiol. 1989 Aug;90(4):1347–1352. doi: 10.1104/pp.90.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takats S. T. Early autoregulation of symbiotic root nodulation in soybeans. Plant Physiol. 1990 Nov;94(3):865–869. doi: 10.1104/pp.94.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]


