Abstract
An extracellular p-coumaroyl esterase produced by the anaerobic fungus Neocallimastix strain MC-2 released p-coumaroyl groups from 0-[5-0-((E)-p-coumaroyl)-alpha-L-arabinofuranosyl]-(1----3)-0-beta -D-xylopyranosyl-(1----4)-D-xylopyranose (PAXX). The esterase was purified 121-fold from culture medium in successive steps involving ultrafiltration column chromatography on S-sepharose and hydroxylapatite, isoelectric focusing, and gel filtration. The native enzyme had an apparent mass of 11 kDa under nondenaturing conditions and a mass of 5.8 kDa under denaturing conditions, suggesting that the enzyme may exist as a dimer. The isoelectric point was 4.7, and the pH optimum was 7.2. The purified esterase had 100 times more activity towards PAXX than towards the analogous feruloyl ester (FAXX). The apparent Km and Vmax of the purified p-coumaroyl esterase for PAXX at pH 7.2 and 40 degrees C were 19.4 microM and 5.1 microM min(-1), respectively. p-Coumaroyl tetrasaccharides isolated from plant cell walls were hydrolyzed at rates similar to that for PAXX, whereas a dimer of PAXX was hydrolyzed at a rate 20-fold lower, yielding 4,4'-dihydroxy-alpha-truxillic acid as an end product. Ethyl and methyl p-coumarates were hydrolyzed at very slow rates, if at all. The purified esterase released p-coumaroyl groups from finely, but not coarsely, ground plant cell walls, and this activity was enhanced by the addition of xylanase and other cell wall-degrading enzymes.
Full text
PDF

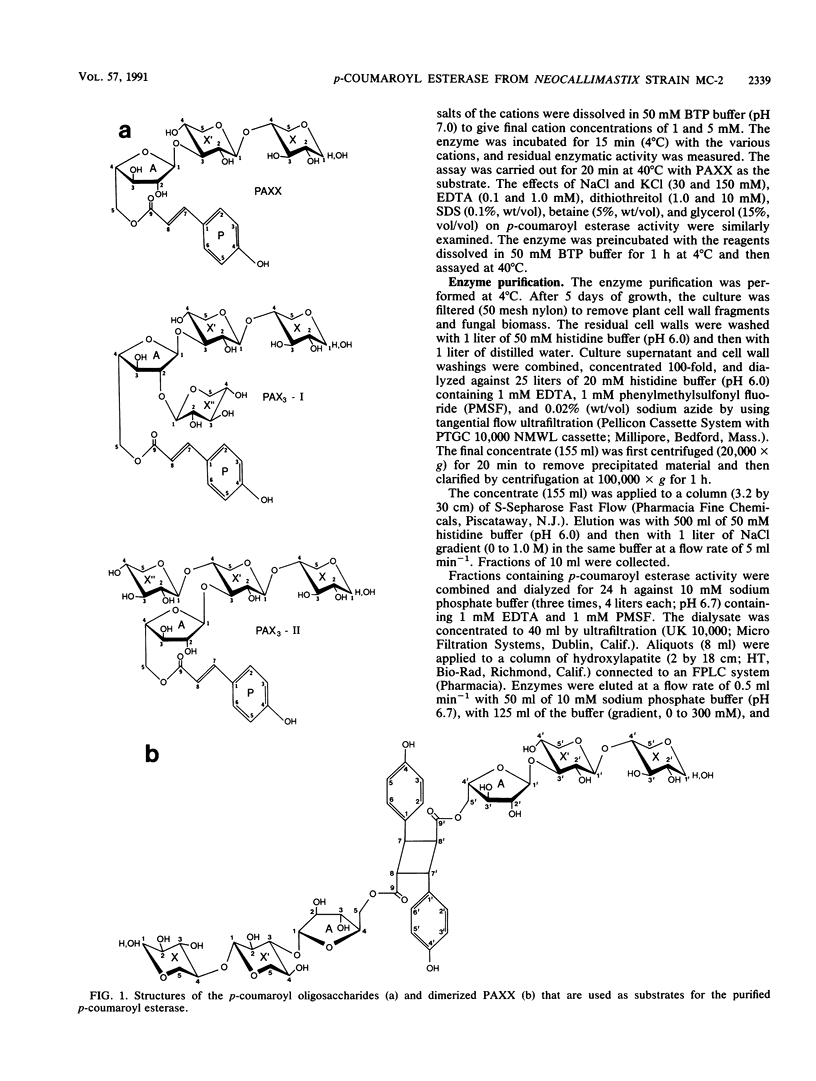
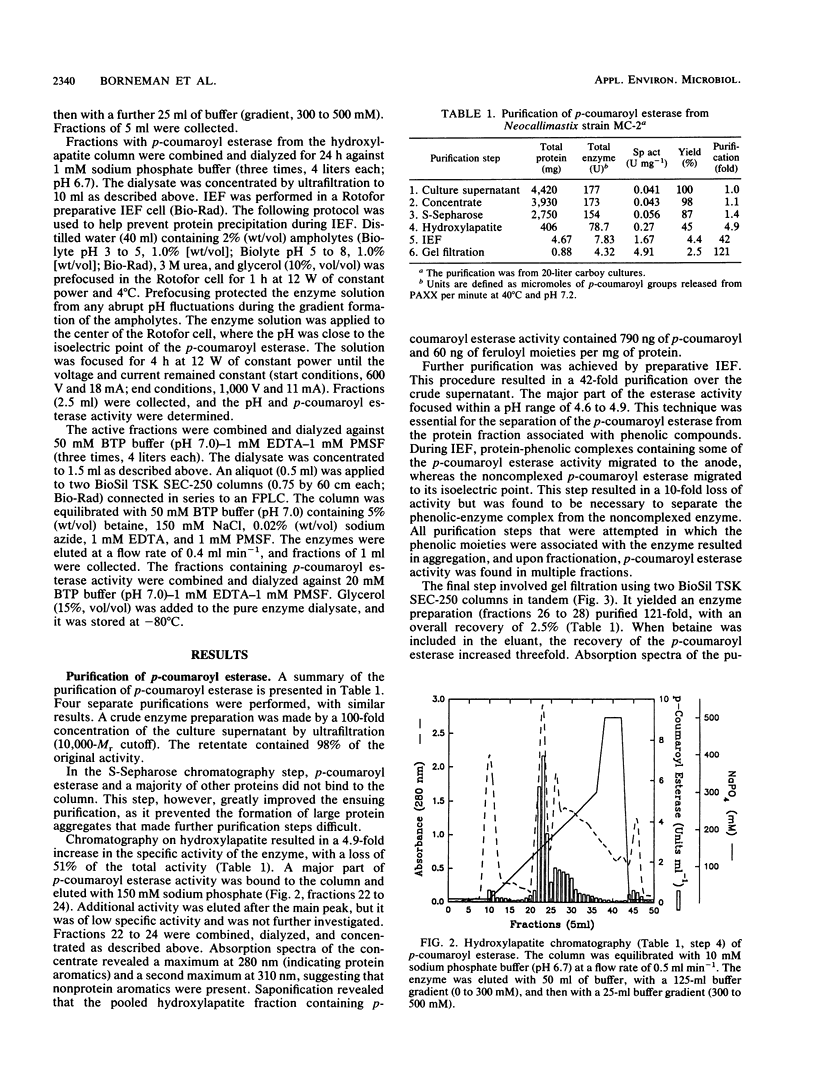
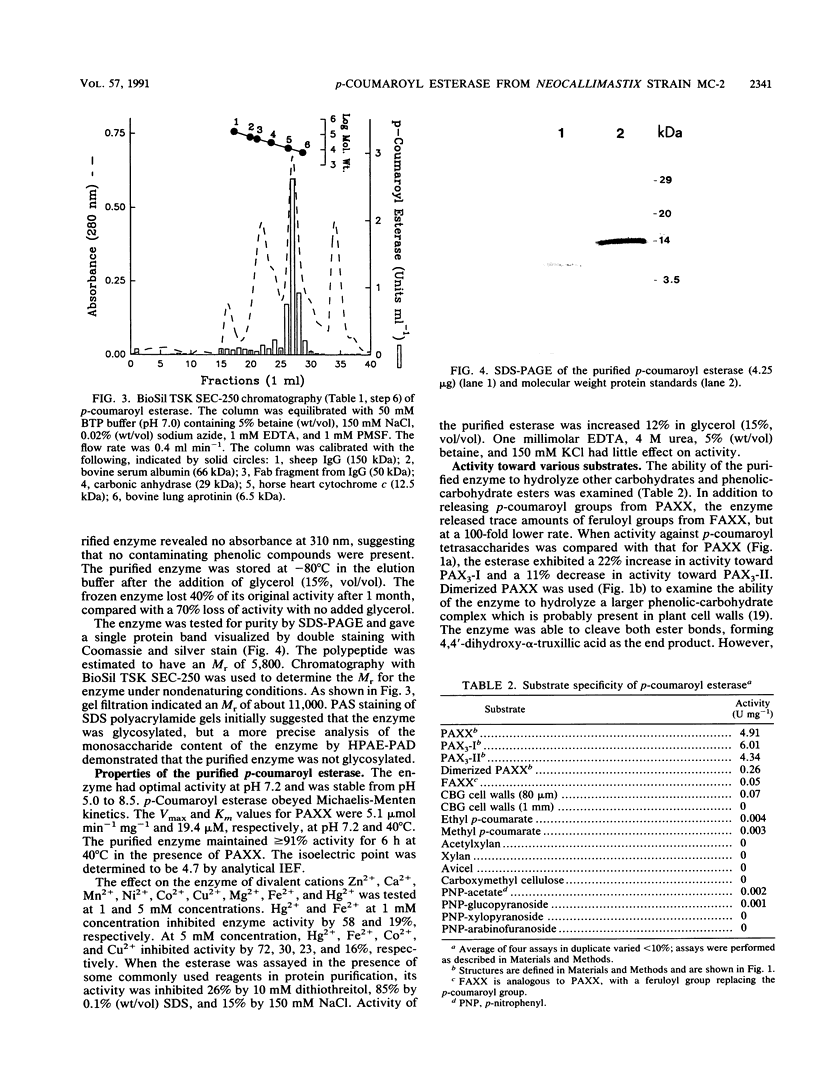



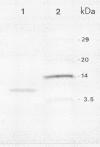
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Rigsby L. L. Mixed fungal populations and lignocellulosic tissue degradation in the bovine rumen. Appl Environ Microbiol. 1987 Sep;53(9):1987–1995. doi: 10.1128/aem.53.9.1987-1995.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman W. S., Akin D. E., Ljungdahl L. G. Fermentation products and plant cell wall-degrading enzymes produced by monocentric and polycentric anaerobic ruminal fungi. Appl Environ Microbiol. 1989 May;55(5):1066–1073. doi: 10.1128/aem.55.5.1066-1073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman W. S., Hartley R. D., Himmelsbach D. S., Ljungdahl L. G. Assay for trans-p-coumaroyl esterase using a specific substrate from plant cell walls. Anal Biochem. 1990 Oct;190(1):129–133. doi: 10.1016/0003-2697(90)90145-y. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchie J., Hay A. J., Lomax J. A. Soluble lignin-carbohydrate complexes from sheep rumen fluid: their composition and structural features. Carbohydr Res. 1988 Jun 15;177:127–151. doi: 10.1016/0008-6215(88)85048-1. [DOI] [PubMed] [Google Scholar]
- De Moreno M. R., Smith J. F., Smith R. V. Silver staining of proteins in polyacrylamide gels: increased sensitivity through a combined Coomassie blue-silver stain procedure. Anal Biochem. 1985 Dec;151(2):466–470. doi: 10.1016/0003-2697(85)90206-4. [DOI] [PubMed] [Google Scholar]
- Donnelly P. K., Crawford D. L. Production by Streptomyces viridosporus T7A of an Enzyme Which Cleaves Aromatic Acids from Lignocellulose. Appl Environ Microbiol. 1988 Sep;54(9):2237–2244. doi: 10.1128/aem.54.9.2237-2244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C. Purification of glycoproteins. Methods Enzymol. 1990;182:529–539. doi: 10.1016/0076-6879(90)82042-z. [DOI] [PubMed] [Google Scholar]
- Jue C. K., Lipke P. N. Determination of reducing sugars in the nanomole range with tetrazolium blue. J Biochem Biophys Methods. 1985 Aug;11(2-3):109–115. doi: 10.1016/0165-022x(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. R., Bilous D. Ferulic Acid Esterase Activity from Schizophyllum commune. Appl Environ Microbiol. 1988 May;54(5):1170–1173. doi: 10.1128/aem.54.5.1170-1173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino M., Davidson W. F., Fredrickson T. N., Hartley J. W., Morse H. C., 3rd Effects of non-MHC loci on resistance to retrovirus-induced immunodeficiency in mice. Immunogenetics. 1991;33(5-6):345–351. doi: 10.1007/BF00216693. [DOI] [PubMed] [Google Scholar]
- McDermid K. P., Mackenzie C. R., Forsberg C. W. Esterase Activities of Fibrobacter succinogenes subsp. succinogenes S85. Appl Environ Microbiol. 1990 Jan;56(1):127–132. doi: 10.1128/aem.56.1.127-132.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Weitzhandler M., Hardy M. Sensitive blotting assay for the detection of glycopeptides in peptide maps. J Chromatogr. 1990 Jun 27;510:225–232. doi: 10.1016/s0021-9673(01)93756-2. [DOI] [PubMed] [Google Scholar]