Abstract
Biodegradation of polyethylene glycols (PEGs) of up to 13,000 to 14,000 molecular weight has been shown to be performed by a river water bacterial isolate (strain JA1001) identified as Pseudomonas stutzeri. A pure culture of strain JA1001 grew on PEG 1000 or PEG 10000 at 0.2% (wt/vol) as a sole source of carbon and energy with a doubling time of 135 or 150 min, respectively. Cultures metabolized 2 g of polymer per liter in less than 24 h and 10 g/liter in less than 72 h. The limit of 13,500 molecular weight in the size of the PEG sustaining growth and the presence of a PEG-oxidative activity in the periplasmic space indicated that PEGs cross the outer membrane and are subsequently metabolized in the periplasm. PEG oxidation was found to be catalyzed by PEG dehydrogenase, an enzyme that has been shown to be a single polypeptide. Characterization of PEG dehydrogenase revealed glyoxylic acid as the product of the PEG-oxidative cleavage. Glyoxylate supported growth by entering the cell and introducing its carbons in the general metabolism via the dicarboxylic acid cycle, as indicated by the ability of strain JA1001 to grow on this compound and the presence of malate synthase, the first enzyme in the pathway, in extracts of PEG-grown cells.
Full text
PDF

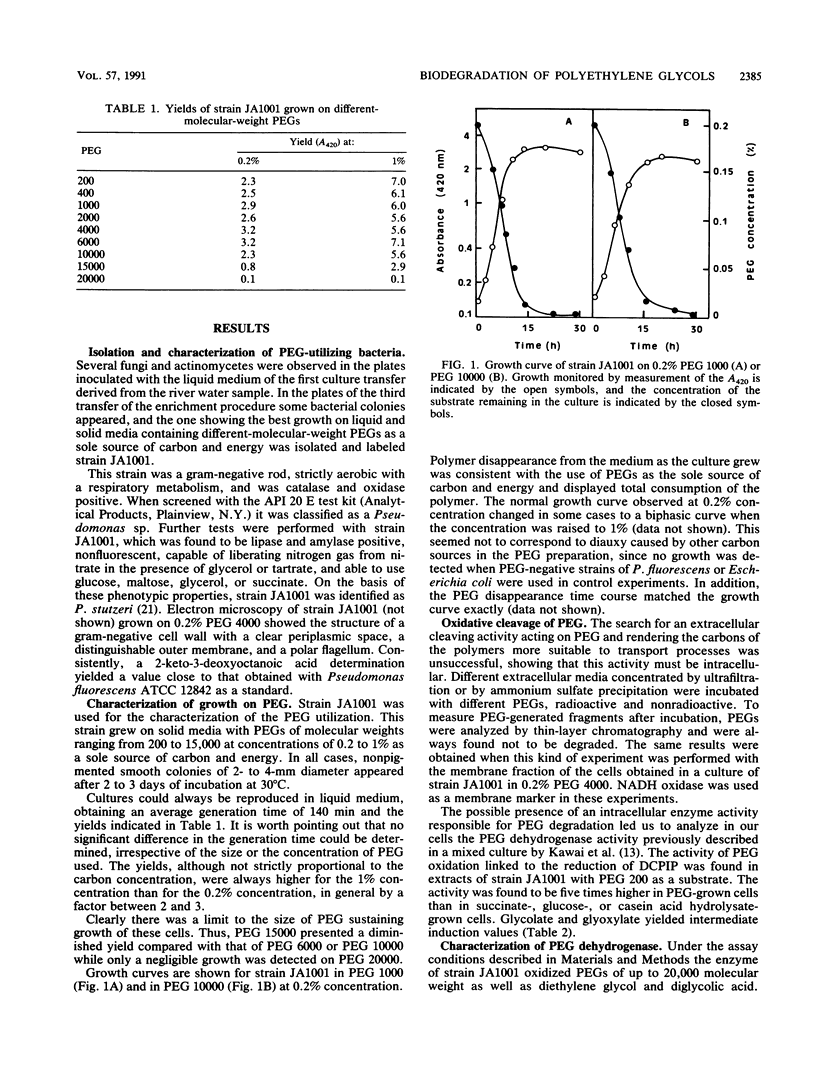
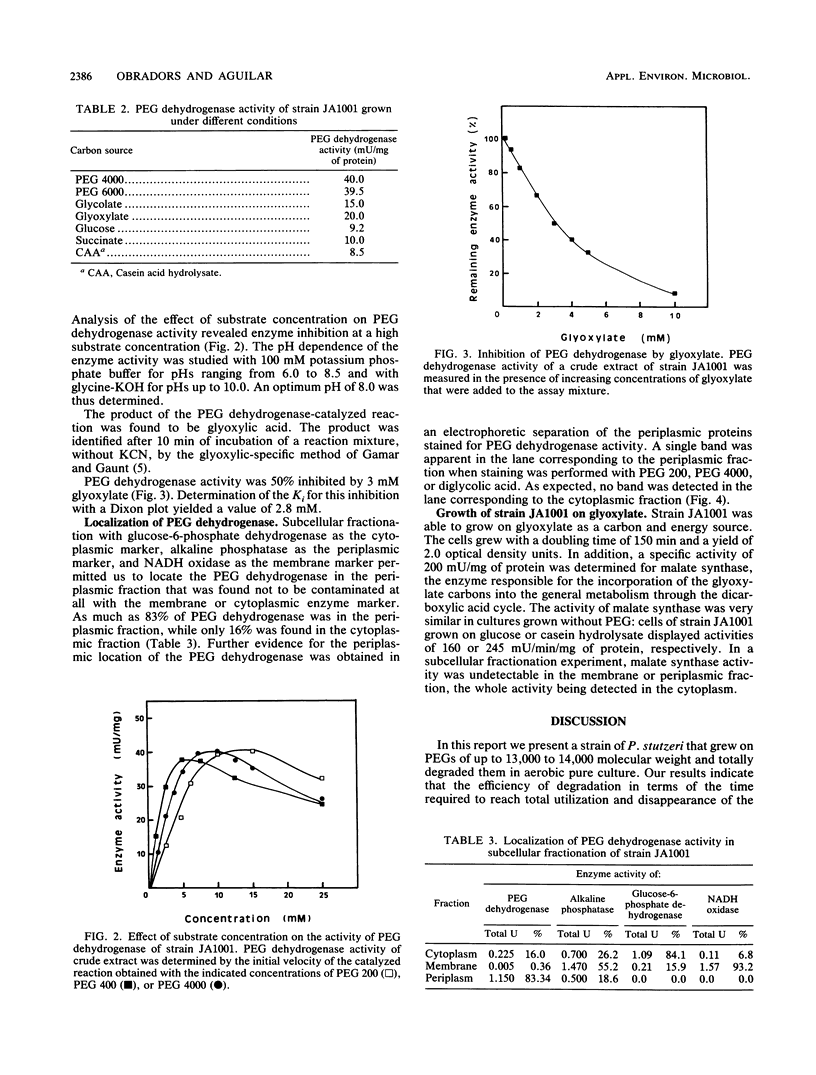


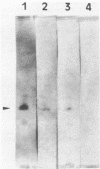
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980 Jan;141(1):199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. F., Tiedje J. M. Degradation of ethylene glycol and polyethylene glycols by methanogenic consortia. Appl Environ Microbiol. 1983 Jul;46(1):185–190. doi: 10.1128/aem.46.1.185-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. F., Tiedje J. M. Metabolism of polyethylene glycol by two anaerobic bacteria, Desulfovibrio desulfuricans and a Bacteroides sp. Appl Environ Microbiol. 1986 Oct;52(4):852–856. doi: 10.1128/aem.52.4.852-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamar Y., Gaunt J. K. Bacterial metabolism of 4-chloro-2-methylphenoxyacetate. Formation of glyoxylate by side-chain cleavage. Biochem J. 1971 May;122(4):527–531. doi: 10.1042/bj1220527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J. R., Alexander M. Microbial degradation of polyethylene glycols. Appl Microbiol. 1975 May;29(5):621–625. doi: 10.1128/am.29.5.621-625.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L. D., Cook K. A., Cain R. B. Microbial degradation of polyethylene glycols. J Appl Bacteriol. 1979 Aug;47(1):75–85. doi: 10.1111/j.1365-2672.1979.tb01171.x. [DOI] [PubMed] [Google Scholar]
- Kawai F., Kimura T., Fukaya M., Tani Y., Ogata K., Ueno T., Fukami H. Bacterial oxidation of polyethylene glycol. Appl Environ Microbiol. 1978 Apr;35(4):679–684. doi: 10.1128/aem.35.4.679-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F., Kimura T., Tani Y., Yamada H., Kurachi M. Purification and characterization of polyethylene glycol dehydrogenase involved in the bacterial metabolism of polyethylene glycol. Appl Environ Microbiol. 1980 Oct;40(4):701–705. doi: 10.1128/aem.40.4.701-705.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F., Yamanaka H. Biodegradation of polyethylene glycol by symbiotic mixed culture (obligate mutualism). Arch Microbiol. 1986 Nov;146(2):125–129. doi: 10.1007/BF00402338. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Bohlander M., Nunn W. D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J Bacteriol. 1980 Aug;143(2):720–725. doi: 10.1128/jb.143.2.720-725.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Stieb M. Fermentative degradation of polyethylene glycol by a strictly anaerobic, gram-negative, nonsporeforming bacterium, Pelobacter venetianus sp. nov. Appl Environ Microbiol. 1983 Jun;45(6):1905–1913. doi: 10.1128/aem.45.6.1905-1913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber J., Wierich P. Metabolites and biodegradation pathways of fatty alcohol ethoxylates in microbial biocenoses of sewage treatment plants. Appl Environ Microbiol. 1985 Mar;49(3):530–537. doi: 10.1128/aem.49.3.530-537.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener S., Schink B. Fermentative degradation of nonionic surfactants and polyethylene glycol by enrichment cultures and by pure cultures of homoacetogenic and propionate-forming bacteria. Appl Environ Microbiol. 1988 Feb;54(2):561–565. doi: 10.1128/aem.54.2.561-565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]