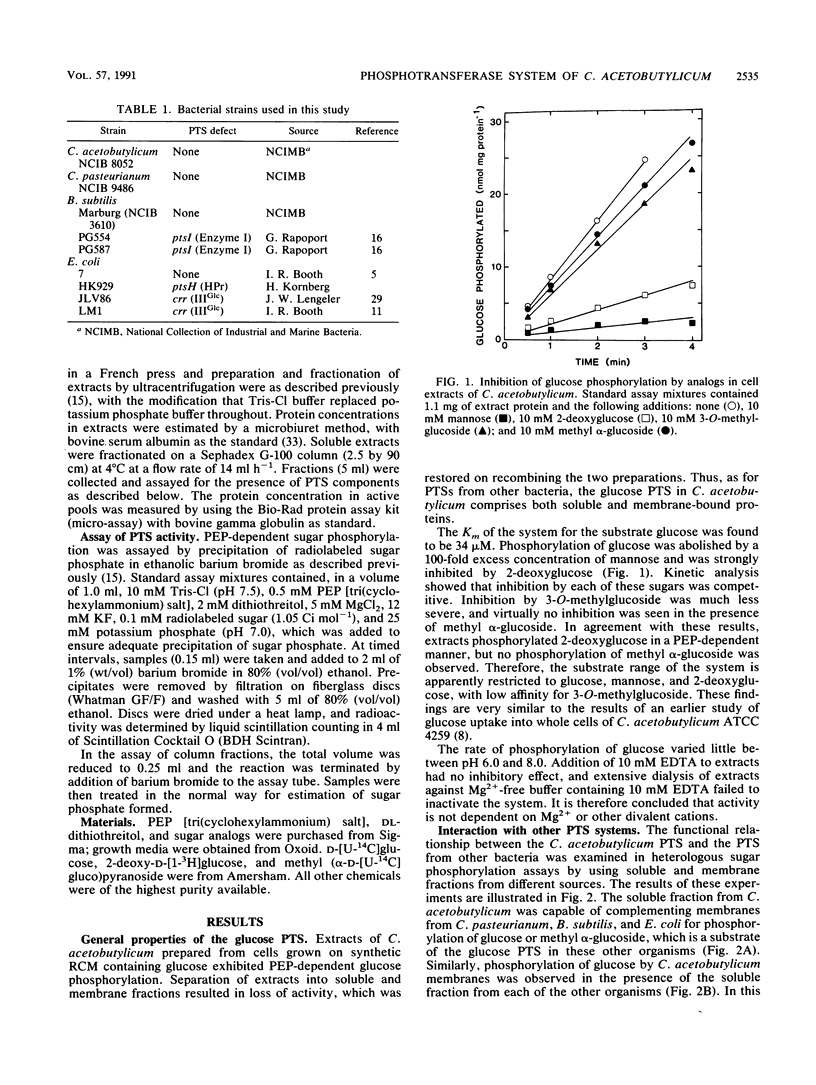
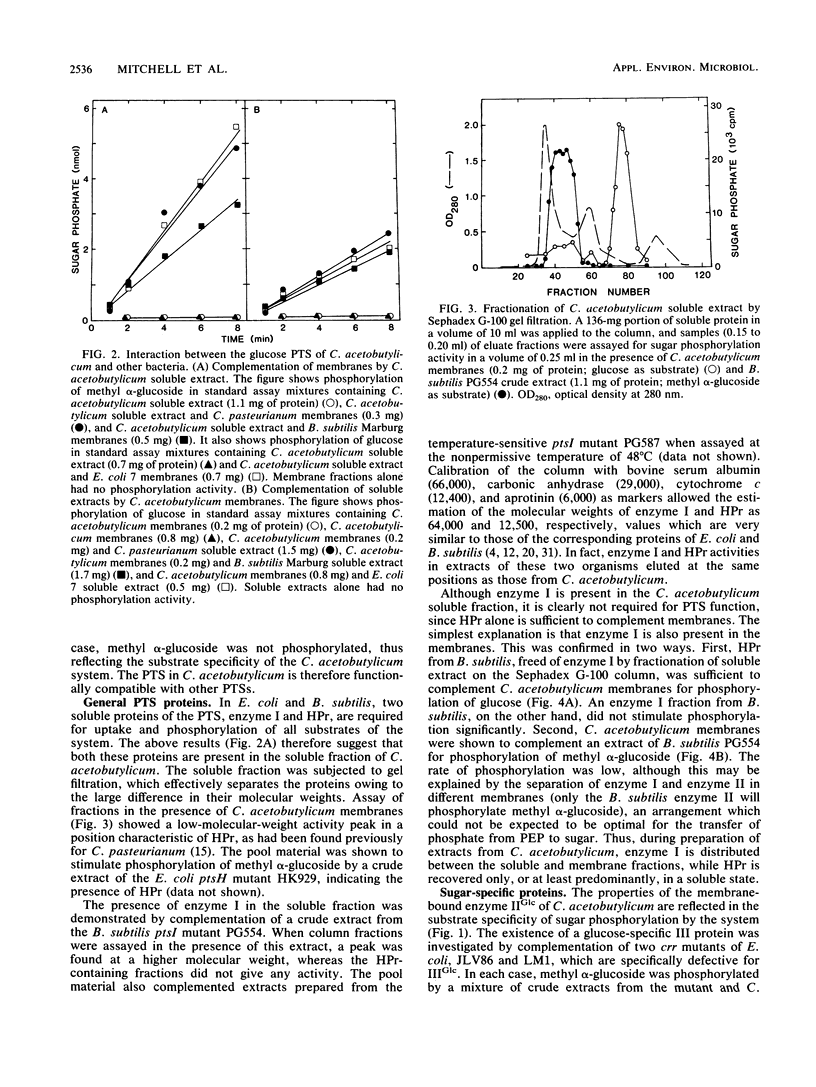
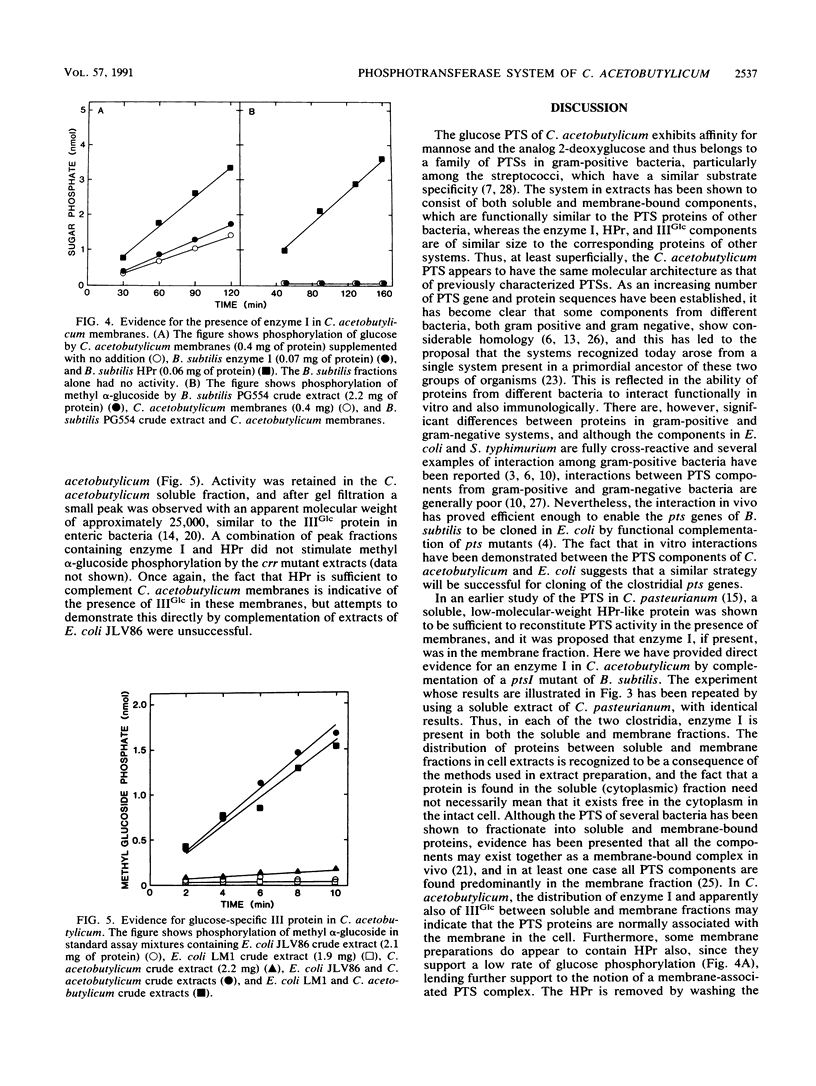
Abstract
The glucose phosphotransferase system (PTS) of Clostridium acetobutylicum was studied by using cell extracts. The system exhibited a Km for glucose of 34 microM, and glucose phosphorylation was inhibited competitively by mannose and 2-deoxyglucose. The analogs 3-O-methylglucoside and methyl alpha-glucoside did not inhibit glucose phosphorylation significantly. Activity showed no dependence on Mg2+ ions or on pH in the range 6.0 to 8.0. The PTS comprised both soluble and membrane-bound proteins, which interacted functionally with the PTSs of Clostridium pasteurianum, Bacillus subtilis, and Escherichia coli. In addition to a membrane-bound enzyme IIGlc, sugar phosphorylation assays in heterologous systems incorporating extracts of pts mutants of other organisms provided evidence for enzyme I, HPr, and IIIGlc components. The HPr was found in the soluble fraction of C. acetobutylicum extracts, whereas enzyme I, and probably also IIIGlc, was present in both the soluble and membrane fractions, suggesting a membrane location in the intact cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. R., Morris J. G. Carbohydrate transport in Clostridium pasteurianum. Biosci Rep. 1982 Jan;2(1):47–53. doi: 10.1007/BF01142198. [DOI] [PubMed] [Google Scholar]
- Chin A. M., Feucht B. U., Saier M. H., Jr Evidence for regulation of gluconeogenesis by the fructose phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1987 Feb;169(2):897–899. doi: 10.1128/jb.169.2.897-899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Tréboul G., Steinmetz M. Phosphoenolpyruvate:sugar phosphotransferase system of Bacillus subtilis: cloning of the region containing the ptsH and ptsI genes and evidence for a crr-like gene. J Bacteriol. 1987 May;169(5):2287–2290. doi: 10.1128/jb.169.5.2287-2290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hutkins R. W., Kashket E. R. Phosphotransferase Activity in Clostridium acetobutylicum from Acidogenic and Solventogenic Phases of Growth. Appl Environ Microbiol. 1986 May;51(5):1121–1123. doi: 10.1128/aem.51.5.1121-1123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüdig H., Hengstenberg W. The bacterial phosphoenolpyruvate dependent phosphotransferase system (PTS): solubilisation and kinetic parameters of the glucose-specific membrane bound enzyme II component of Streptococcus faecalis. FEBS Lett. 1980 May 19;114(1):103–106. doi: 10.1016/0014-5793(80)80869-6. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer H. R., Hengstenberg W., Rösch P., Muss P., Bernsmann P., Engelmann R., Dörschug M., Deutscher J. HPr proteins of different microorganisms studied by hydrogen-1 high-resolution nuclear magnetic resonance: similarities of structures and mechanisms. Biochemistry. 1982 Jun 8;21(12):2879–2885. doi: 10.1021/bi00541a012. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Marquet M., Creignou M. C., Dedonder R. The phosphoenolpyruvate : methyl-alpha-D-glucoside phosphotransferase system in Bacillus subtilis Marburg 168 : purification and identification of the phosphocarrier protein (HPr). Biochimie. 1976;58(4):435–441. doi: 10.1016/s0300-9084(76)80254-4. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Niaudet B., Gay P., Dedonder R. Identification of the structural gene of the PEP-phosphotransferase enzyme I in Bacillus subtilis Marburg. Mol Gen Genet. 1975;136(4):337–349. doi: 10.1007/BF00341718. [DOI] [PubMed] [Google Scholar]
- Patni N. J., Alexander J. K. Catabolism of fructose and mannitol in Clostridium thermocellum: presence of phosphoenolpyruvate: fructose phosphotransferase, fructose 1-phosphate kinase, phosphoenolpyruvate: mannitol phosphotransferase, and mannitol 1-phosphate dehydrogenase in cell extracts. J Bacteriol. 1971 Jan;105(1):226–231. doi: 10.1128/jb.105.1.226-231.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Hengstenberg W., Saier M. H., Jr Properties of ATP-dependent protein kinase from Streptococcus pyogenes that phosphorylates a seryl residue in HPr, a phosphocarrier protein of the phosphotransferase system. J Bacteriol. 1984 Oct;160(1):333–340. doi: 10.1128/jb.160.1.333-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Saffen D. W., Presper K. A., Doering T. L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Molecular cloning and structural analysis of the Escherichia coli ptsH, ptsI, and crr genes. J Biol Chem. 1987 Nov 25;262(33):16241–16253. [PubMed] [Google Scholar]
- Saier M. H., Jr, Cox D. F., Feucht B. U., Novotny M. J. Evidence for the functional association of enzyme I and HPr of the phosphoenolpyruvate-sugar phosphotransferase system with the membrane in sealed vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):231–238. doi: 10.1002/jcb.1982.240180210. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Grenier F. C., Lee C. A., Waygood E. B. Evidence for the evolutionary relatedness of the proteins of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. J Cell Biochem. 1985;27(1):43–56. doi: 10.1002/jcb.240270106. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Staley J. T. Phosphoenolpyruvate:sugar phosphotransferase system in Ancalomicrobium adetum. J Bacteriol. 1977 Aug;131(2):716–718. doi: 10.1128/jb.131.2.716-718.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Yamada M., Erni B., Suda K., Lengeler J., Ebner R., Argos P., Rak B., Schnetz K., Lee C. A. Sugar permeases of the bacterial phosphoenolpyruvate-dependent phosphotransferase system: sequence comparisons. FASEB J. 1988 Mar 1;2(3):199–208. doi: 10.1096/fasebj.2.3.2832233. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Vadeboncoeur C. Structure and properties of the phosphoenolpyruvate: glucose phosphotransferase system of oral streptococci. Can J Microbiol. 1984 Apr;30(4):495–502. doi: 10.1139/m84-073. [DOI] [PubMed] [Google Scholar]
- Vogler A. P., Broekhuizen C. P., Schuitema A., Lengeler J. W., Postma P. W. Suppression of IIIGlc-defects by enzymes IINag and IIBgl of the PEP:carbohydrate phosphotransferase system. Mol Microbiol. 1988 Nov;2(6):719–726. doi: 10.1111/j.1365-2958.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Steeves T. Enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. Purification to homogeneity and some properties. Can J Biochem. 1980 Jan;58(1):40–48. doi: 10.1139/o80-006. [DOI] [PubMed] [Google Scholar]
- Yamada M., Feucht B. U., Saier M. H., Jr Regulation of gluconeogenesis by the glucitol enzyme III of the phosphotransferase system in Escherichia coli. J Bacteriol. 1987 Dec;169(12):5416–5422. doi: 10.1128/jb.169.12.5416-5422.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hugo H., Gottschalk G. Distribution of 1-phosphofructokinase and PEP:fructose phosphotransferase activity in Clostridia. FEBS Lett. 1974 Sep 15;46(1):106–108. doi: 10.1016/0014-5793(74)80345-5. [DOI] [PubMed] [Google Scholar]


