Abstract
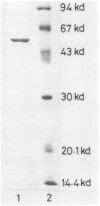
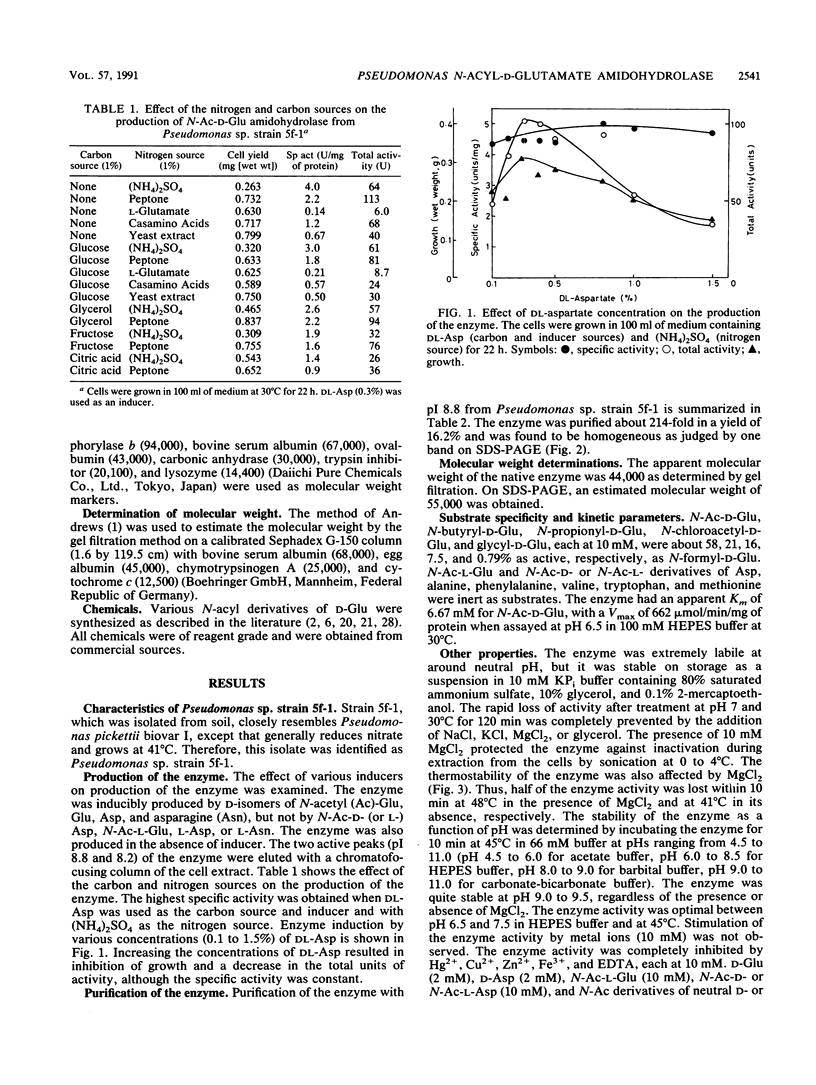
N-Acyl-D-glutamate amidohydrolase from Pseudomonas sp. strain 5f-1 was inducibly produced by D isomers of N-acetylglutamate, glutamate, aspartate, and asparagine. The enzyme has been purified to homogeneity by DEAE-cellulose, (NH4)2SO4 fractionation, and chromatofocusing followed by gel filtration on a Sephadex G-100 column. The enzyme was a monomer with molecular weight of 55,000. The enzyme activity was optimal at pH 6.5 to 7.5 and 45 degrees C. The isoelectric point and the pH stability were 8.8 and 9.0, respectively. N-Formyl, N-acetyl, N-butyryl, N-propionyl, N-chloroacetyl derivatives of D-glutamate and glycyl-D-glutamate were substrates for the enzyme. At pH 6.5 in 100 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer at 30 degrees C, a Km of 6.67 mM and a Vmax of 662 mumol/min/mg of protein for N-acetyl-D-glutamate were obtained. None of the metal ions stimulated the enzyme activity. Na+, K+, Mg2+, and Ba2+ acted as stabilizers. Hg2+, Cu2+, Zn2+, Fe3+, and EDTA were strongly inhibitory.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The control of the enzymes degrading histidine and related imidazolyl derivates in Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):423–433. doi: 10.1042/bj1320423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Früh H., Leisinger T. Properties and localization of N-acetylglutamate deacetylase from Pseudomonas aeruginosa. J Gen Microbiol. 1981 Jul;125(1):1–10. doi: 10.1099/00221287-125-1-1. [DOI] [PubMed] [Google Scholar]
- Fujii N., Muraoka S., Harada K. Purification and characterization of a protein containing D-aspartic acid in bovine lens. Biochim Biophys Acta. 1989 Dec 21;999(3):239–242. doi: 10.1016/0167-4838(89)90003-4. [DOI] [PubMed] [Google Scholar]
- Helfman P. M., Bada J. L. Aspartic acid racemization in tooth enamel from living humans. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2891–2894. doi: 10.1073/pnas.72.8.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Mulfinger L. M., Phillips A. T. Purification and properties of formylglutamate amidohydrolase from Pseudomonas putida. J Bacteriol. 1987 Oct;169(10):4696–4702. doi: 10.1128/jb.169.10.4696-4702.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMEDA Y., TOYOURA E., YAMAZOE H., KIMURA Y., YASUDA Y. Hydrolysis and metabolism by soil bacteria of benzoyl derivatives of D- and L-forms of some amino-acids. Nature. 1952 Nov 22;170(4334):888–889. doi: 10.1038/170888a0. [DOI] [PubMed] [Google Scholar]
- Kameda Y., Hase T., Kanatomo S., Kita Y. Studies on acylase activity and micro-organisms. XXVI. Purification from AAA 6029 (Pseudomonase sp). Chem Pharm Bull (Tokyo) 1978 Sep;26(9):2698–2704. doi: 10.1248/cpb.26.2698. [DOI] [PubMed] [Google Scholar]
- Kendrick K. E., Wheelis M. L. Histidine dissimilation in Streptomyces coelicolor. J Gen Microbiol. 1982 Sep;128(9):2029–2040. doi: 10.1099/00221287-128-9-2029. [DOI] [PubMed] [Google Scholar]
- Kubo K., Ishikura T., Fukagawa Y. Deacetylation of PS-5, a new beta-lactam compound. II. Separation and purification of L- and D-amino acid acylases from Pseudomonas sp. 1158. J Antibiot (Tokyo) 1980 Jun;33(6):550–555. doi: 10.7164/antibiotics.33.550. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man E. H., Sandhouse M. E., Burg J., Fisher G. H. Accumulation of D-aspartic acid with age in the human brain. Science. 1983 Jun 24;220(4604):1407–1408. doi: 10.1126/science.6857259. [DOI] [PubMed] [Google Scholar]
- Moriguchi M., Ideta K. Production of d-Aminoacylase from Alcaligenes denitrificans subsp. xylosoxydans MI-4. Appl Environ Microbiol. 1988 Nov;54(11):2767–2770. doi: 10.1128/aem.54.11.2767-2770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO K. R., BIRNBAUM S. M., KINGSLEY R. B., GREENSTEIN J. P. Enzymatic susceptibility of corresponding chloroacetyl- and glycyl-L-amino acids. J Biol Chem. 1952 Oct;198(2):507–524. [PubMed] [Google Scholar]
- ROBINSON D. S., BIRNBAUM S. M., GREENSTEIN J. P. Purification and properties of an aminopeptidase from kidney cellular particulates. J Biol Chem. 1953 May;202(1):1–26. [PubMed] [Google Scholar]
- Tsai Y. C., Tseng C. P., Hsiao K. M., Chen L. Y. Production and Purification of d-Aminoacylase from Alcaligenes denitrificans and Taxonomic Study of the Strain. Appl Environ Microbiol. 1988 Apr;54(4):984–989. doi: 10.1128/aem.54.4.984-989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]