Abstract
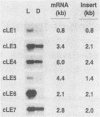
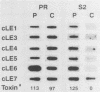
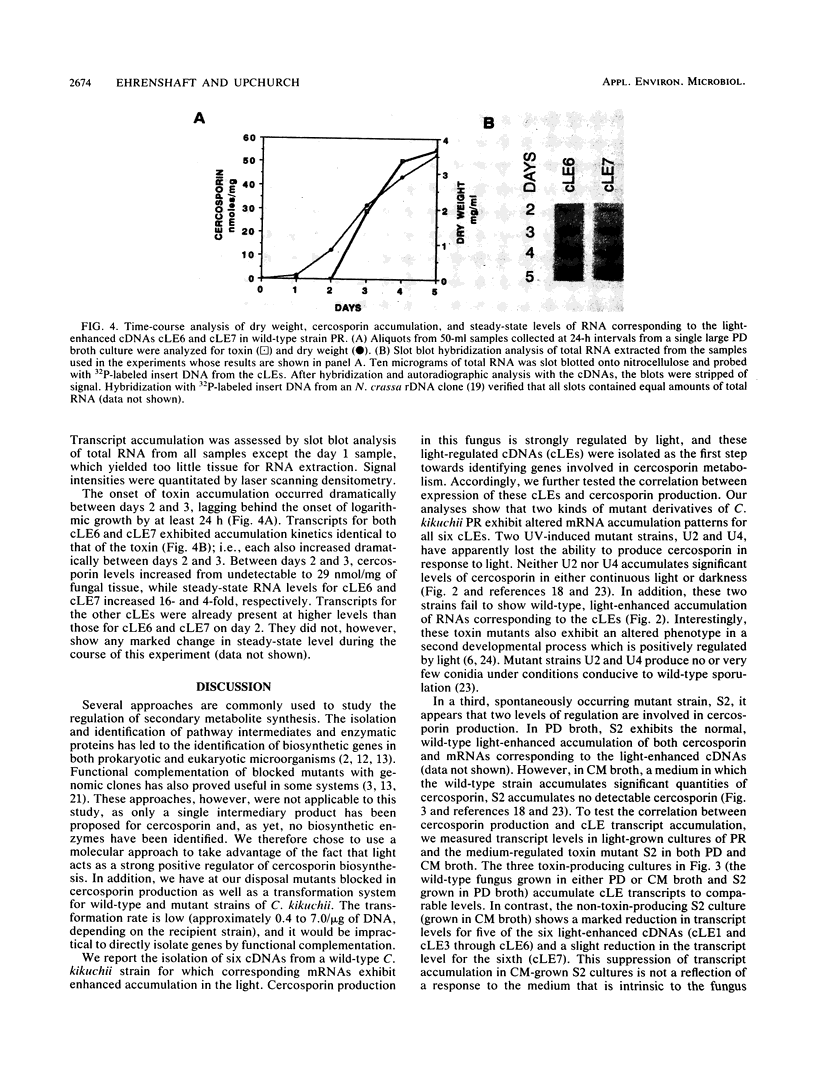
Cercospora kikuchii is a fungal pathogen of soybeans which produces a photosensitizing phytotoxic polyketide metabolite, cercosporin. Cercosporin synthesis in culture is modulated by several environmental factors. In addition to the light requirement for toxin action, cercosporin biosynthesis is also highly light regulated. As a first step towards identifying genes involved in cercosporin regulation and biosynthesis, we have used subtractive hybridization to isolate light-enhanced cDNA clones. Six distinct cDNA clones representing genes from a wild-type C. kikuchii strain for which transcript accumulation is positively regulated by light were isolated. To assess the relationship of these light-enhanced cDNAs to cercosporin biosynthesis, we compared corresponding steady-state RNA levels in the wild type and in three mutant strains altered in toxin biosynthesis. Two of the mutant C. kikuchii strains which fail to accumulate cercosporin in response to light also fail to exhibit light-enhanced accumulation of transcripts corresponding to all six light-enhanced cDNAs. Cercosporin accumulation in the third mutant strain, S2, is regulated by medium composition as well as light. S2 fails to accumulate cercosporin in complete medium, a medium which allows significant cercosporin accumulation by the wild-type strain. When cultured in complete medium, this mutant strain also fails to show the wild-type, light-enhanced accumulation of transcripts corresponding to five of the six light-enhanced cDNAs. Kinetic analysis demonstrated that transcript accumulation for two of the six light-enhanced cDNAs strongly paralleled cercosporin accumulation in light-grown wild-type culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J., Ripka S., Siegner A., Schiltz E., Schweizer E. The multifunctional 6-methylsalicylic acid synthase gene of Penicillium patulum. Its gene structure relative to that of other polyketide synthases. Eur J Biochem. 1990 Sep 11;192(2):487–498. doi: 10.1111/j.1432-1033.1990.tb19252.x. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Biró S., Motamedi H., Collins J. F., Hutchinson C. R. Analysis of the nucleotide sequence of the Streptomyces glaucescens tcmI genes provides key information about the enzymology of polyketide antibiotic biosynthesis. EMBO J. 1989 Sep;8(9):2727–2736. doi: 10.1002/j.1460-2075.1989.tb08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub M. E., Hangarter R. P. Light-induced production of singlet oxygen and superoxide by the fungal toxin, cercosporin. Plant Physiol. 1983 Nov;73(3):855–857. doi: 10.1104/pp.73.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Beremand P. D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989 Jun 30;79(1):131–138. doi: 10.1016/0378-1119(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Wagner S., Rodland K. D., Feinbaum R. L., Russell J. P., Bret-Harte M. S., Free S. J., Metzenberg R. L. Organization of the ribosomal ribonucleic acid genes in various wild-type strains and wild-collected strains of Neurospora. Mol Gen Genet. 1984;196(2):275–282. doi: 10.1007/BF00328060. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Bull J. H., Hodgson J. E., Ward J. M., Browne P., Brown J., Barton B., Earl A. J., Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990 Mar;9(3):741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. D., Galili G., Larkins B. A., Gelvin S. B. The synthesis of a 19 kilodalton zein protein in transgenic petunia plants. Plant Physiol. 1988 Dec;88(4):1002–1007. doi: 10.1104/pp.88.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]