Abstract
Genetic systems for study of Aeromicrobium erythreum, a gram-positive, G + C-rich (72%) bacterium with the capacity for erythromycin biosynthesis, are described. High-copy-number plasmids suitable as gene cloning vectors include derivatives of the Streptomyces plasmids pIJ101, pVE1, and pJV1. pIJ101 derivatives with missense substitutions at the rep gene BamHI site do not replicate in A. erythreum. Ethyl methanesulfonate treatment generated several amino acid auxotrophs and non-erythromycin-producing (Ery-) strains. Using the Ery- strain AR1807 as a recipient for plasmid-directed integrative recombination, the chromosomal ermR gene (encoding 23S rRNA methyltransferase) was disrupted. Phenotypic characterizations demonstrated that ermR is the sole determinant of macrolide antibiotic resistance in A. erythreum.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. R., Bruton C. J., Butler M. J., Chater K. F., Harris J. E., Hopwood D. A. Properties of in vitro recombinant derivatives of pJV1, a multi-copy plasmid from Streptomyces phaeochromogenes. J Gen Microbiol. 1986 Aug;132(8):2071–2078. doi: 10.1099/00221287-132-8-2071. [DOI] [PubMed] [Google Scholar]
- Dubnau D. Translational attenuation: the regulation of bacterial resistance to the macrolide-lincosamide-streptogramin B antibiotics. CRC Crit Rev Biochem. 1984;16(2):103–132. doi: 10.3109/10409238409102300. [DOI] [PubMed] [Google Scholar]
- Epp J. K., Burgett S. G., Schoner B. E. Cloning and nucleotide sequence of a carbomycin-resistance gene from Streptomyces thermotolerans. Gene. 1987;53(1):73–83. doi: 10.1016/0378-1119(87)90094-1. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Bibb M. J., Chater K. F., Kieser T. Plasmid and phage vectors for gene cloning and analysis in Streptomyces. Methods Enzymol. 1987;153:116–166. doi: 10.1016/0076-6879(87)53052-x. [DOI] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimiya S., Weisblum B. Translational attenuation control of ermSF, an inducible resistance determinant encoding rRNA N-methyltransferase from Streptomyces fradiae. J Bacteriol. 1988 Apr;170(4):1800–1811. doi: 10.1128/jb.170.4.1800-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall K. J., Cohen S. N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988 Oct;170(10):4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Larson J. L., Hershberger C. L. The minimal replicon of a streptomycete plasmid produces an ultrahigh level of plasmid DNA. Plasmid. 1986 May;15(3):199–209. doi: 10.1016/0147-619x(86)90038-7. [DOI] [PubMed] [Google Scholar]
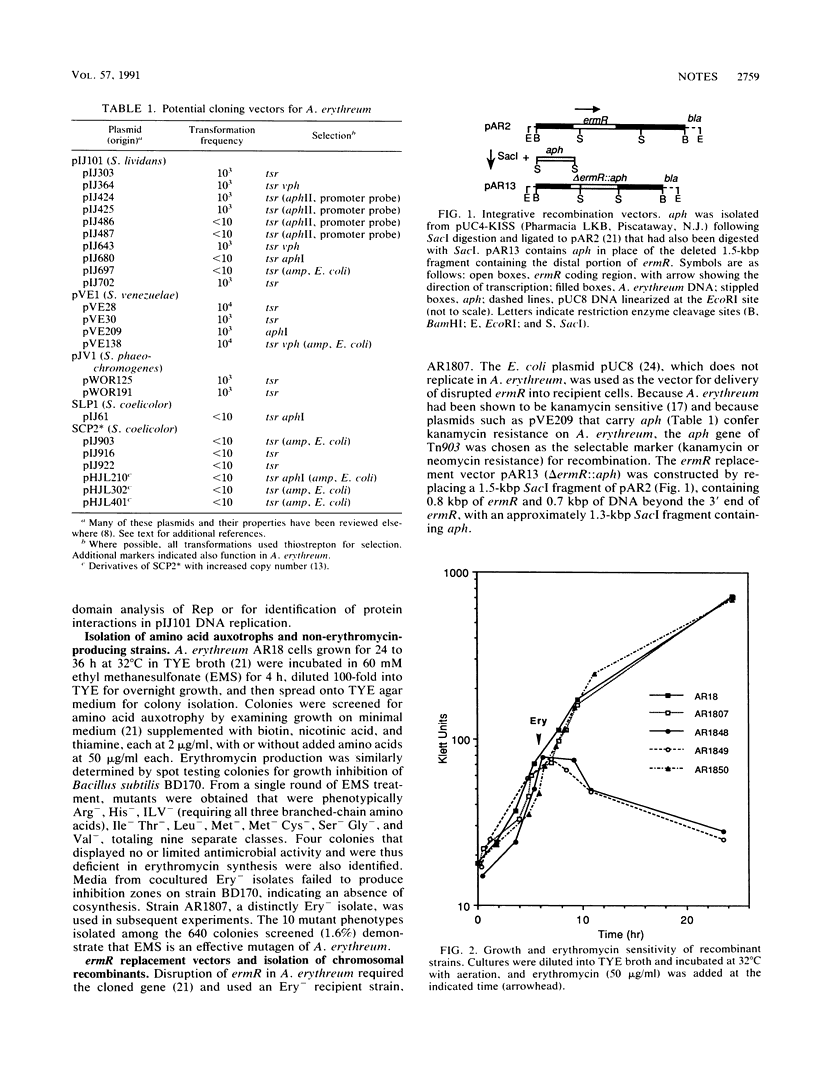
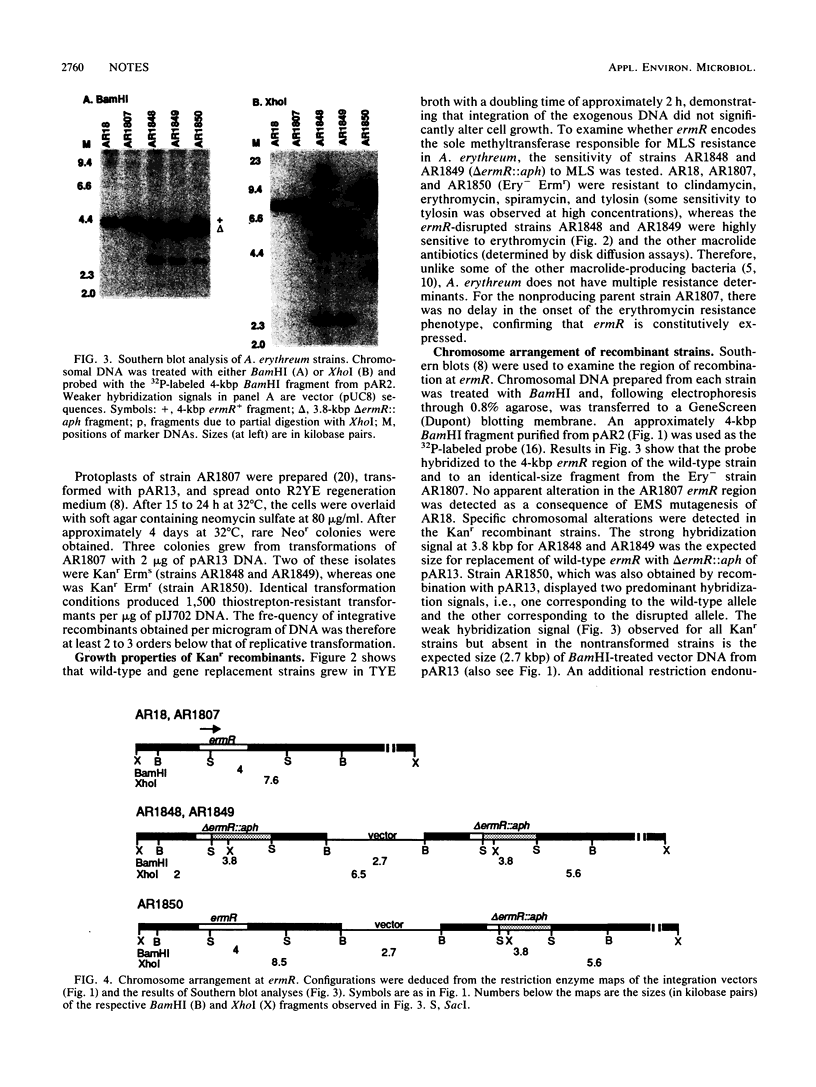
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- MacNeil T., Gibbons P. H. Characterization of the Streptomyces plasmid pVE1. Plasmid. 1986 Nov;16(3):182–194. doi: 10.1016/0147-619x(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Miller E. S., Woese C. R., Brenner S. Description of the erythromycin-producing bacterium Arthrobacter sp. strain NRRL B-3381 as Aeromicrobium erythreum gen. nov., sp. nov. Int J Syst Bacteriol. 1991 Jul;41(3):363–368. doi: 10.1099/00207713-41-3-363. [DOI] [PubMed] [Google Scholar]
- Paradiso M. J., Roberts G., Streicher S. L., Goldberg R. B. Characterization of suppressible mutations in the viomycin phosphotransferase gene of the Streptomyces enteric plasmid pVE138. J Bacteriol. 1987 Mar;169(3):1325–1327. doi: 10.1128/jb.169.3.1325-1327.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Barnett L., Brenner S. Transformation of Arthrobacter and studies on the transcription of the Arthrobacter ermA gene in Streptomyces lividans and Escherichia coli. Biochem J. 1987 Apr 15;243(2):431–436. doi: 10.1042/bj2430431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Hudson G. S., Brenner S. An erythromycin-resistance gene from an erythromycin-producing strain of Arthrobacter sp. Gene. 1985;35(3):259–270. doi: 10.1016/0378-1119(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Uchiyama H., Weisblum B. N-Methyl transferase of Streptomyces erythraeus that confers resistance to the macrolide-lincosamide-streptogramin B antibiotics: amino acid sequence and its homology to cognate R-factor enzymes from pathogenic bacilli and cocci. Gene. 1985;38(1-3):103–110. doi: 10.1016/0378-1119(85)90208-2. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Losick R. The use of a chromosome integration vector to map erythromycin resistance and production genes in Saccharopolyspora erythraea (Streptomyces erythraeus). Gene. 1988 Sep 7;68(2):173–180. doi: 10.1016/0378-1119(88)90019-4. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalacain M., Cundliffe E. Methylation of 23S rRNA caused by tlrA (ermSF), a tylosin resistance determinant from Streptomyces fradiae. J Bacteriol. 1989 Aug;171(8):4254–4260. doi: 10.1128/jb.171.8.4254-4260.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]