Abstract
R-plasmids originally isolated from Streptococcus pyogenes(pAC1,pAM15346), Streptococcus agalactiae(pIP501), and Streptococcus faecalis(pAM beta 1) were shown to be self-transferable on filter membranes from S. faecalis JH2-2 to Staphylococcus aureus recipients. The nonconjugative plasmid pAM alpha 1 was mobilized into S. aureus by pAM beta 1. Once in S. aureus, conjugative R-plasmids could be transferred to a second S. aureus recipient or back into S. faecalis. Determinants for chloramphenicol, clindamycin, erythromycin, and tetracycline resistances present on these streptococcal plasmids were expressed in S. aureus. Agarose gel electrophoresis, dye-buoyant centrifugation, and restriction endonuclease digestion showed that the plasmids were maintained intact as self-replicating elements in S. aureus recipients.
Full text
PDF

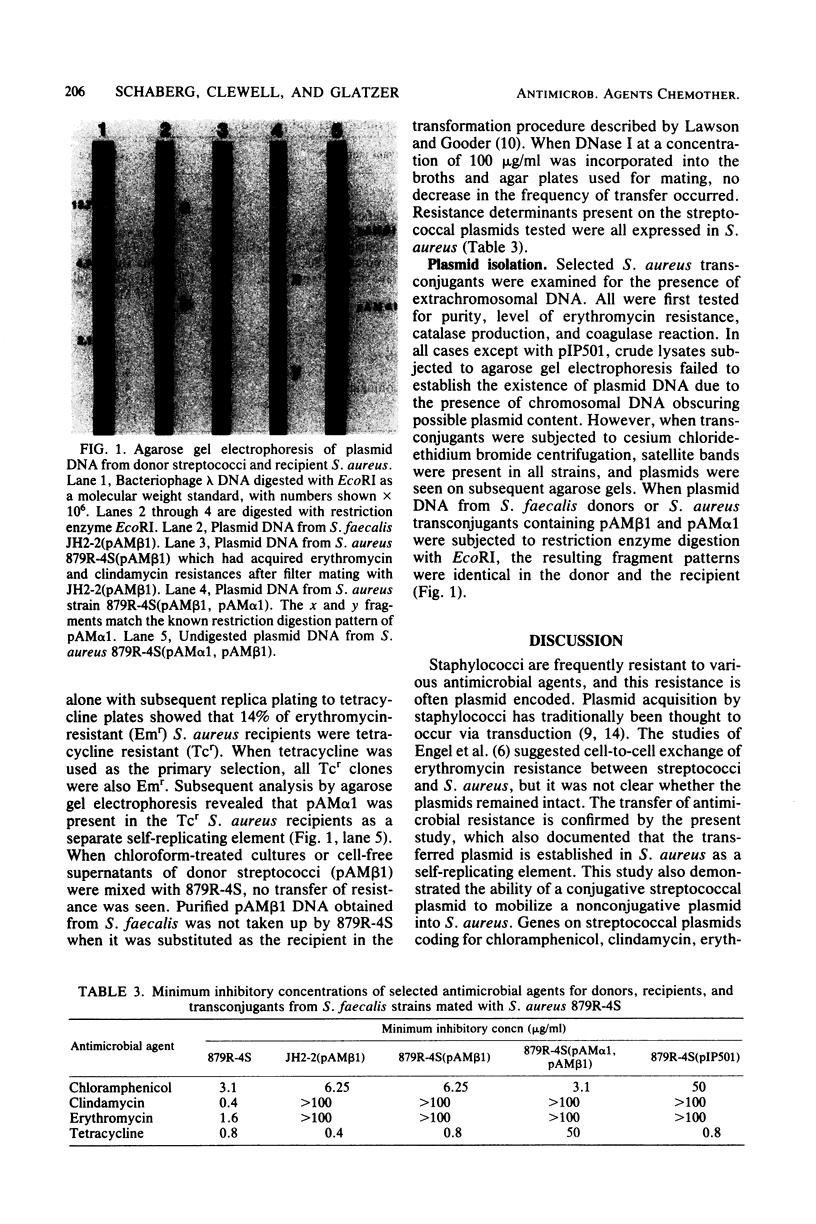

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel H. W., Soedirman N., Rost J. A., van Leeuwen W. J., van Embden J. D. Transferability of macrolide, lincomycin, and streptogramin resistances between group A, B, and D streptococci, Streptococcus pneumoniae, and Staphylococcus aureus. J Bacteriol. 1980 May;142(2):407–413. doi: 10.1128/jb.142.2.407-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H., Bieth G., Chabbert Y. A. R plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1976 Nov;10(5):795–801. doi: 10.1128/aac.10.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Richmond M. H. The genetic basis of antibiotic resistance in S. aureus: the importance of gene transfer in the evolution of this organism in the hospital environment. Ann N Y Acad Sci. 1974 Jul 31;236(0):395–412. doi: 10.1111/j.1749-6632.1974.tb41506.x. [DOI] [PubMed] [Google Scholar]
- Lawson J. W., Gooder H. Growth and development of competence in the group H streptococci. J Bacteriol. 1970 Jun;102(3):820–825. doi: 10.1128/jb.102.3.820-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol Gen Genet. 1974;135(4):349–367. doi: 10.1007/BF00271149. [DOI] [PubMed] [Google Scholar]
- Meijers J. A., Winkler K. C., Stobberingh E. E. Resistance transfer in mixed cultures of Staphylococcus aureus. J Med Microbiol. 1981 Feb;14(1):21–39. doi: 10.1099/00222615-14-1-21. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Semel J. D., Trenholme G. M., Levin S. Gentamicin- and clindamycin-resistant Staphylococcus aureus. Am J Med Sci. 1980 Jul-Aug;280(1):4–9. doi: 10.1097/00000441-198007000-00001. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. Improved method for conjugative transfer by filter mating of Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):457–459. doi: 10.1128/jb.144.1.457-459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobberingh E. E., Winkler K. C. Restriction-deficient mutants of Staphylococcus aureus. J Gen Microbiol. 1977 Apr;99(2):359–367. doi: 10.1099/00221287-99-2-359. [DOI] [PubMed] [Google Scholar]



