Abstract
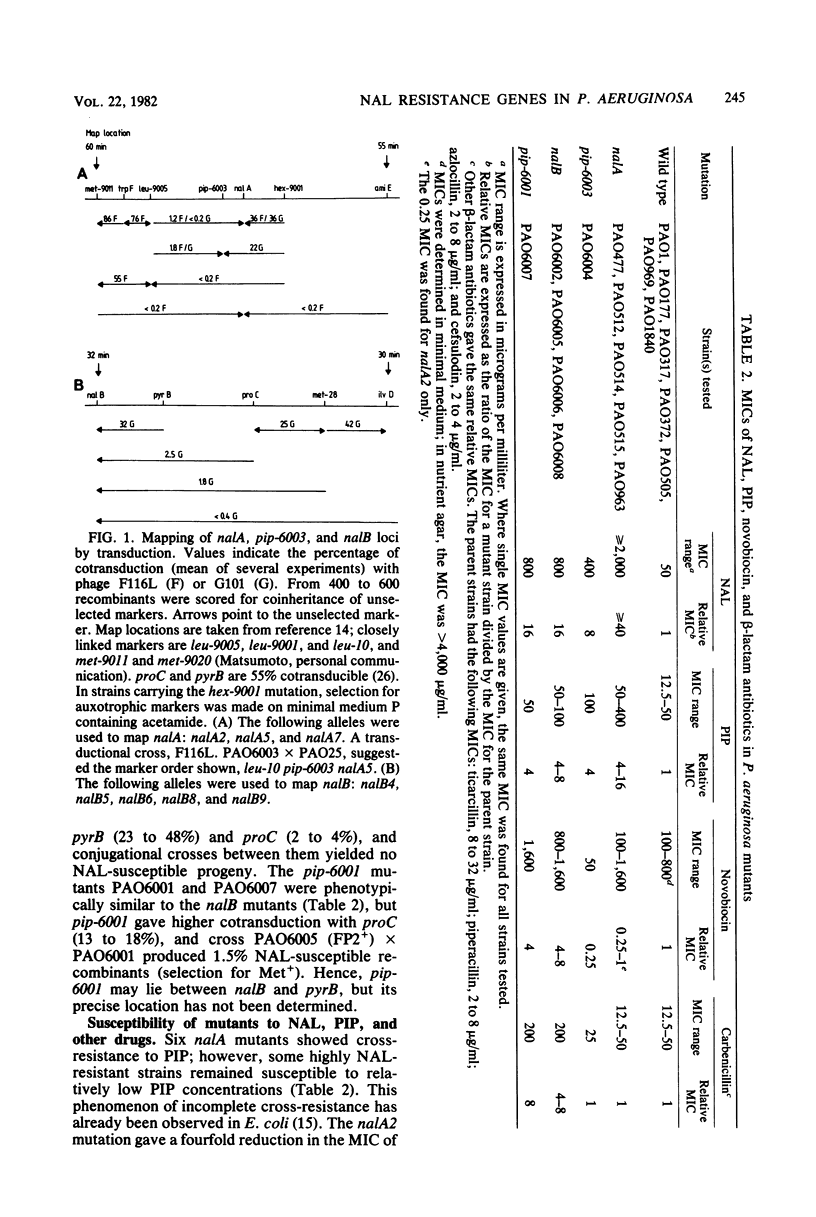
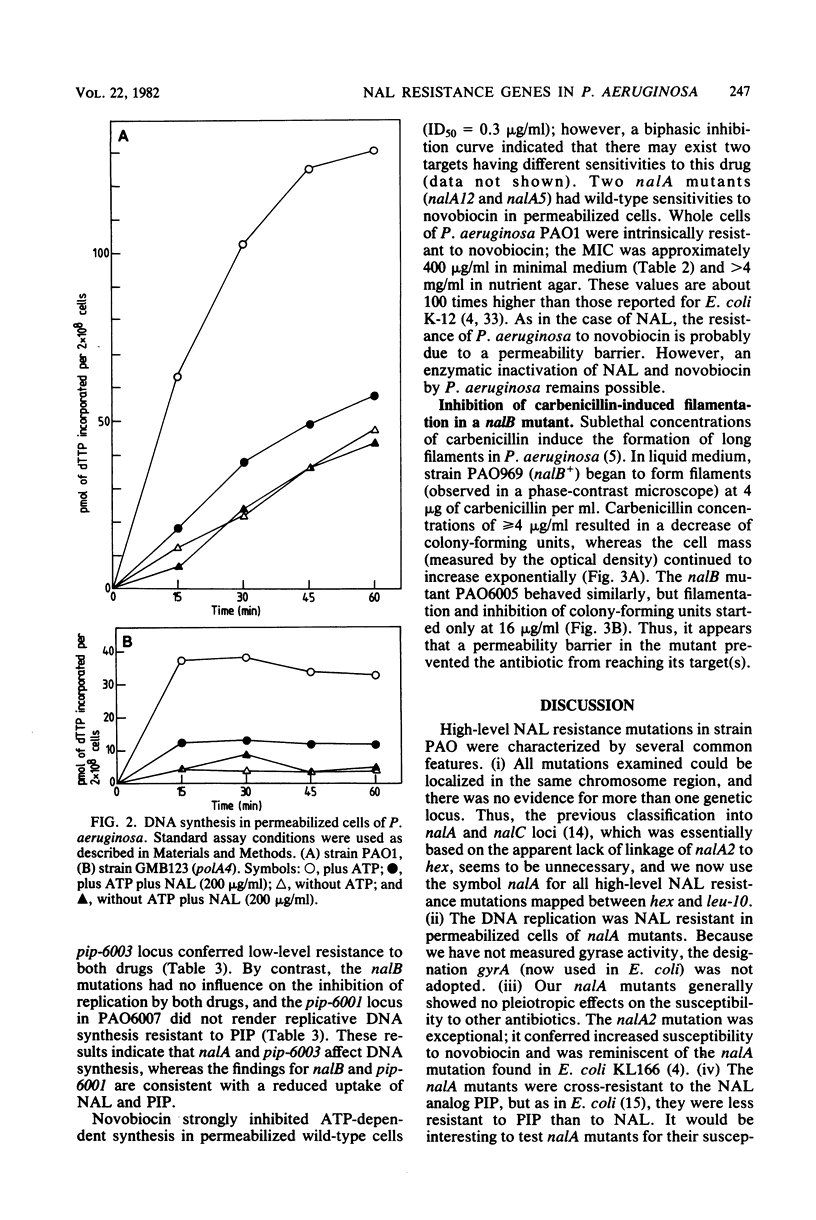
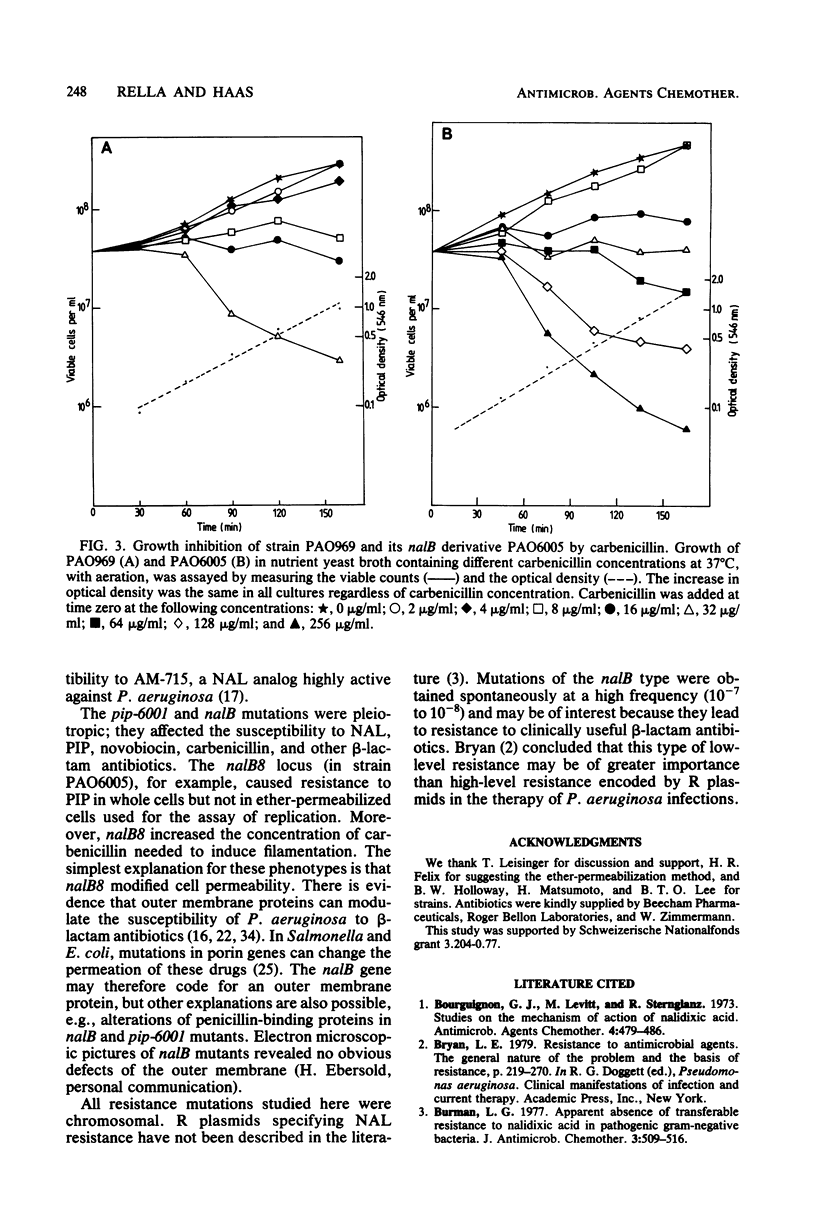
Resistance to high concentrations of nalidixic acid in Pseudomonas aeruginosa PAO was due to mutations in one locus designated nalA, which was mapped by transduction between hex-9001 and leu-10. The nalA mutants were cross-resistant to pipemidic acid, a nalidixic acid analog, at relatively low concentrations. Replicative DNA synthesis was resistant to both drugs in permeabilized cells of nalA mutants. A locus coding for low-level resistance to nalidixic acid, nalB, was cotransducible with pyrB, proC, and met-28. The nalB mutants were also resistant to low levels of pipemidic acid, novobiocin, and beta-lactam antibiotics (e.g., carbenicillin, azlocillin, and cefsulodin), but not to other drugs, such as gentamicin, rifampin, kanamycin, or tetracycline. In nalB mutants, DNA replication showed wild-type sensitivity to nalidixic acid, whereas carbenicillin-induced filamentation required higher drug levels than in the wild-type strain. Thus, nalB mutations appear to decrease cell permeability to some antibiotics. The sensitivity of replicative DNA synthesis to nalidixic acid and novobiocin was very similar in P. aeruginosa and Escherichia coli; by contrast, the concentrations of these drugs needed to inhibit growth of P. aeruginosa were higher than those reported for E. coli by one or two orders of magnitude.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burman L. G. Apparent absence of transferable resistance to nalidixic acid in pathogenic Gram-negative bacteria. J Antimicrob Chemother. 1977 Sep;3(5):509–516. doi: 10.1093/jac/3.5.509. [DOI] [PubMed] [Google Scholar]
- Chao L. An unusual interaction between the target of nalidixic acid and novobiocin. Nature. 1978 Jan 26;271(5643):385–386. doi: 10.1038/271385a0. [DOI] [PubMed] [Google Scholar]
- Ellis L. F., Herron D. K., Preston D. A., Simmons L. K., Schlegel R. A. Evaluation of antibiotic efficacy using electron microscopy: morphological effects of guanylureido cephalosporin, chlorobenzoylureido cephalosporin, BL-P1654, and carbenicillin on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Feb;9(2):334–342. doi: 10.1128/aac.9.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Fisher L. M., O'Dea M. H. DNA gyrase: purification and catalytic properties of a fragment of gyrase B protein. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6289–6293. doi: 10.1073/pnas.76.12.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Ohmori H., Tomizawa J. DNA gyrase and DNA supercoiling. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):35–40. doi: 10.1101/sqb.1979.043.01.007. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. Chromosome mobilization by the R plasmid R68.45: a tool in Pseudomonas genetics. Mol Gen Genet. 1978 Jan 17;158(3):229–237. doi: 10.1007/BF00267194. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W., Schamböck A., Leisinger T. The genetic organization of arginine biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1977 Jul 7;154(1):7–22. doi: 10.1007/BF00265571. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Ohue T., Yamagishi J., Nakamura S., Shimizu M. Mode of incomplete cross-resistance among pipemidic, piromidic, and nalidixic acids. Antimicrob Agents Chemother. 1978 Aug;14(2):240–245. doi: 10.1128/aac.14.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Govan J. W., Fyfe J. A., Costerton J. W. Heterogeneity of antibiotic resistance in mucoid isolates of Pseudomonas aeruginosa obtained from cystic fibrosis patients: role of outer membrane proteins. Antimicrob Agents Chemother. 1981 Jun;19(6):1056–1063. doi: 10.1128/aac.19.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Milazzo F. H. Susceptibility of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa strain PAO to dyes, detergents, and antibiotics. Antimicrob Agents Chemother. 1978 Mar;13(3):494–499. doi: 10.1128/aac.13.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach P. R., Kung A. H., Lee B. T. Mutants of Pseudomonas aeruginosa deficient in DNA polymerase I. Mutat Res. 1976 Dec;41(2-3):391–394. doi: 10.1016/0027-5107(76)90112-3. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Nuchamowitz Y., Rubinstein E. Insensitivity of peptidoglycan biosynthetic reactions to beta-lactam antibiotics in a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):687–695. doi: 10.1128/aac.19.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagate T., Komatsu T., Izawa A., Ohmura S., Namiki S., Mitsuhashi S. Mode of action of a new nalidixic acid derivative, AB206. Antimicrob Agents Chemother. 1980 May;17(5):763–769. doi: 10.1128/aac.17.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa. Genet Res. 1972 Jun;19(3):251–260. doi: 10.1017/s0016672300014518. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Takase Y., Nakamura S., Katae H., Minami A. Pipemidic acid, a new antibacterial agent active against Pseudomonas aeruginosa: in vitro properties. Antimicrob Agents Chemother. 1975 Aug;8(2):132–138. doi: 10.1128/aac.8.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L. Letters to the editor: Novobiocin-a specific inhibitor of semiconservative DNA replication in permeabilized Escherichia coli cells. J Mol Biol. 1975 Jul 25;96(1):201–205. doi: 10.1016/0022-2836(75)90191-6. [DOI] [PubMed] [Google Scholar]
- Voellym R., Leisinger T. Role of 4-aminobutyrate aminotransferase in the arginine metabolism of Pseudomonas aeruginosa. J Bacteriol. 1976 Dec;128(3):722–729. doi: 10.1128/jb.128.3.722-729.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Watson J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa PAT. J Bacteriol. 1978 Mar;133(3):1113–1125. doi: 10.1128/jb.133.3.1113-1125.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. M., Holloway B. W. Linkage map of Pseudomonas aeruginosa PAT. J Bacteriol. 1978 Nov;136(2):507–521. doi: 10.1128/jb.136.2.507-521.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]


