Abstract
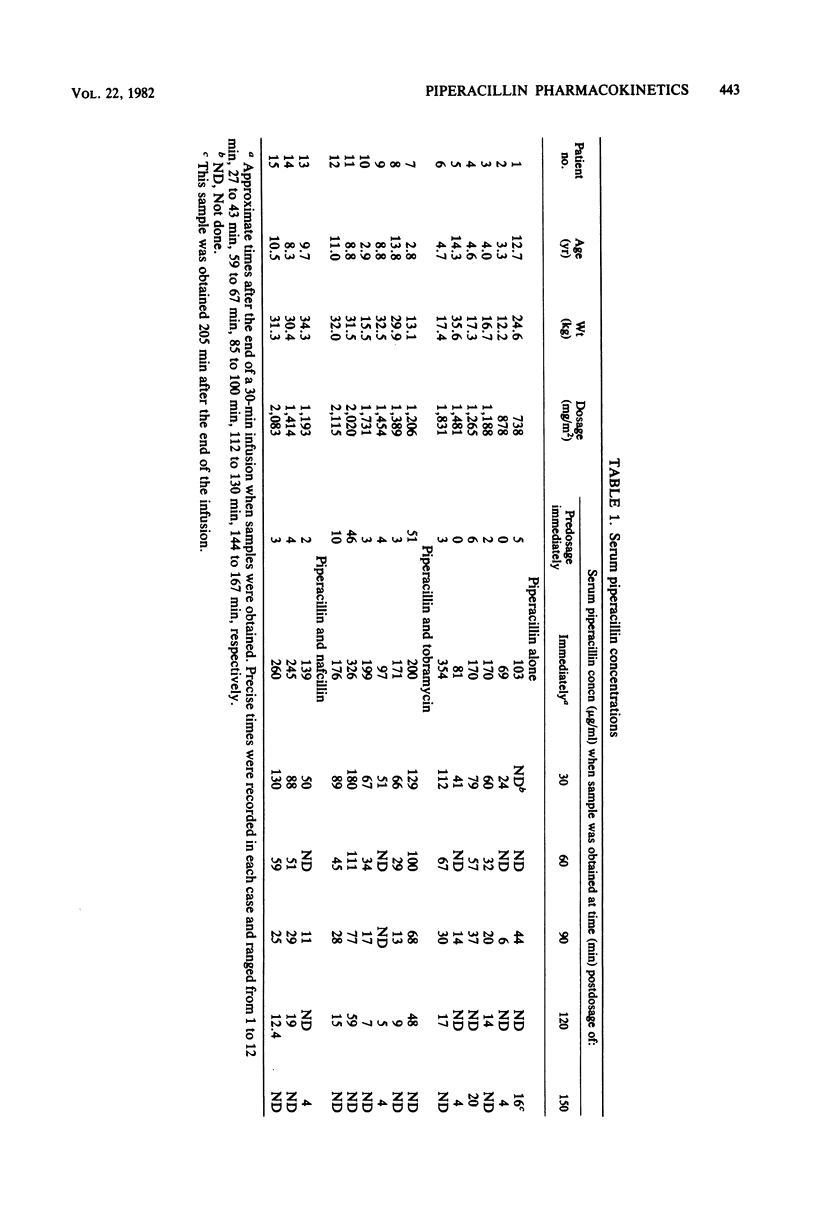
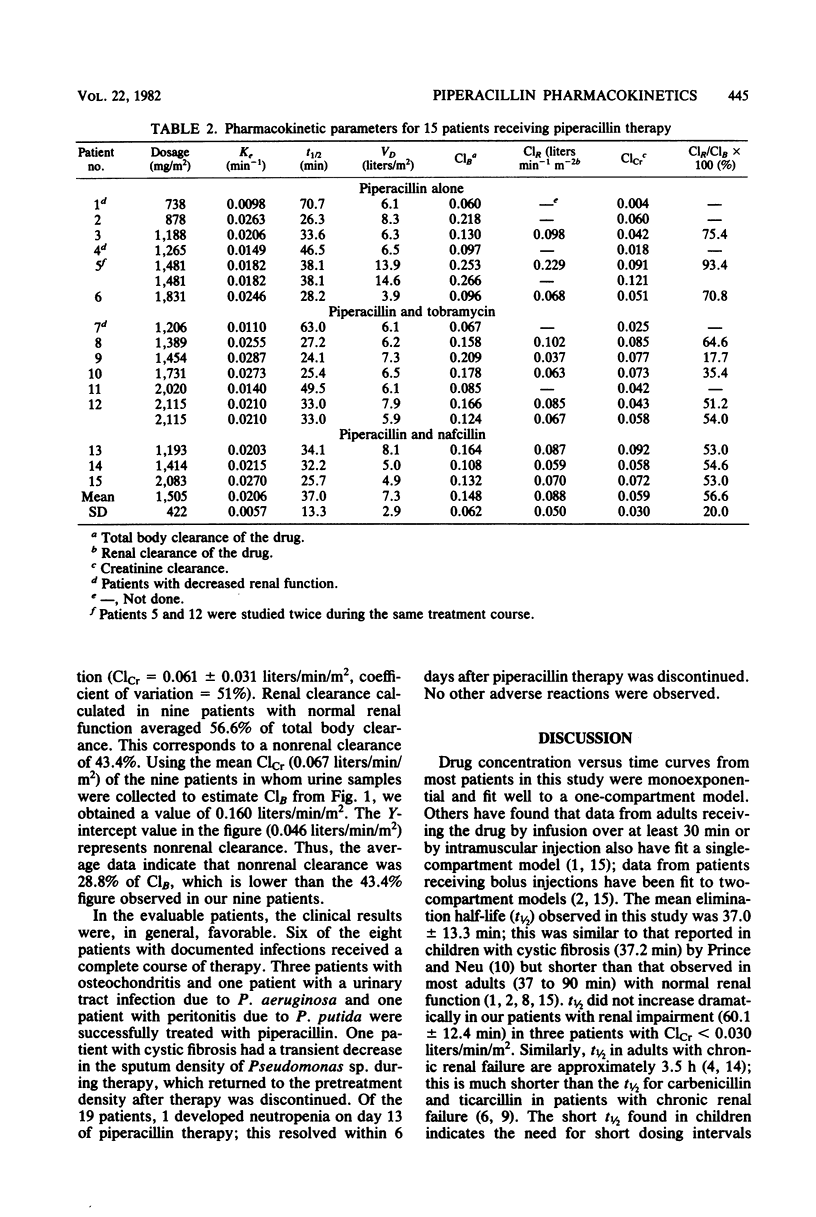
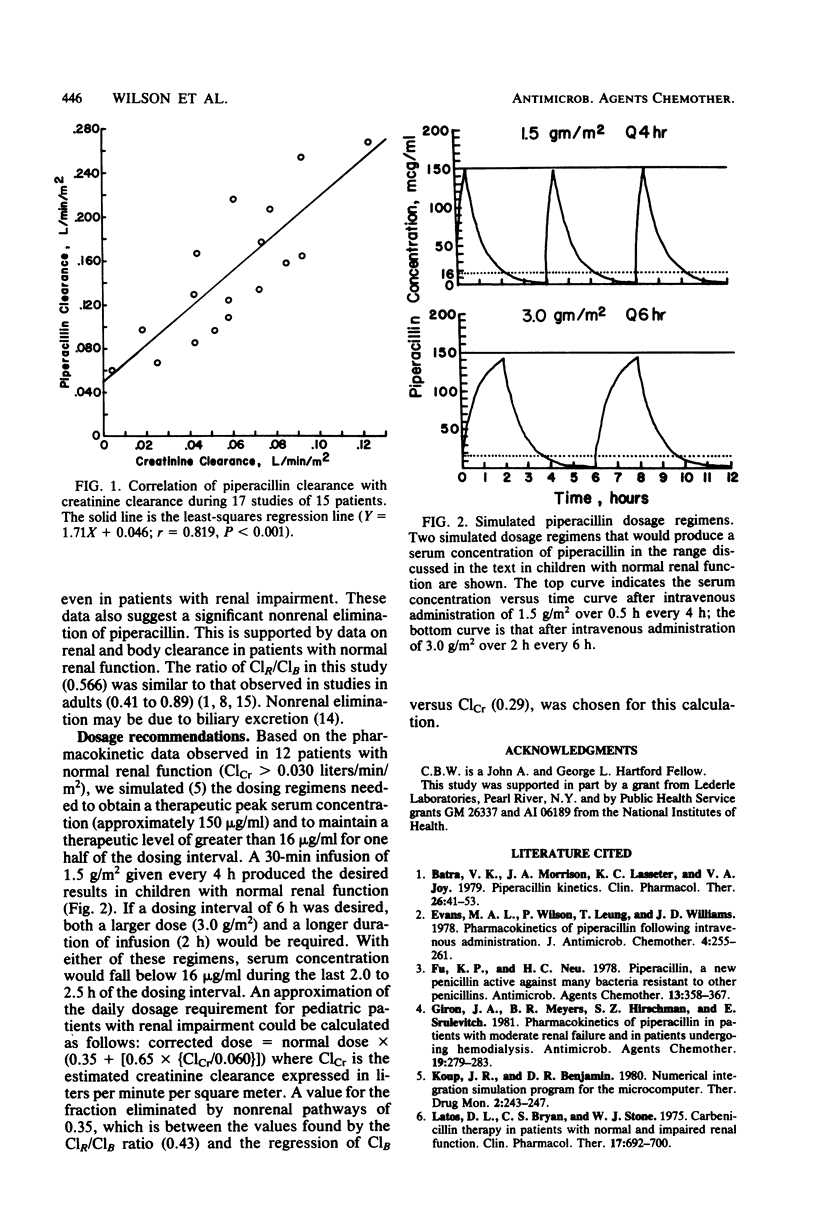
The pharmacokinetics of piperacillin were studied in 15 pediatric patients (age range, 3.3 to 14.3 years). Piperacillin was administered in a dosage of 1.5 +/- 0.4 g/m2 (mean +/- standard deviation) every 4 to 6 h. Peak serum concentrations ranged from 69 to 354 micrograms/ml. The mean elimination half-life was 37.0 +/- 13.3 min, which is shorter than that observed in most adults with normal renal function. The mean elimination half-life in three patients with renal impairment was 60.1 +/- 12.4 min, and the mean ratio of renal clearance to total clearance was 0.57. These results suggest a significant nonrenal elimination of piperacillin. Based on these data, a dosage of 1.5 g/m2 given as a 30-min infusion every 4 h is suggested for children with normal renal function. For patients with renal impairment, the daily dosage could be calculated as follows: corrected dose = normal dose x (0.35 + [0.65 x (ClCr/0.06)]), where ClCr is the creatinine clearance expressed as liters per minute per square meter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra V. K., Morrison J. A., Lasseter K. C., Joy V. A. Piperacillin kinetics. Clin Pharmacol Ther. 1979 Jul;26(1):41–53. doi: 10.1002/cpt197926141. [DOI] [PubMed] [Google Scholar]
- Evans M. A., Wilson P., Leung T., Williams J. D. Pharmacokinetics of piperacillin following intravenous administration. J Antimicrob Chemother. 1978 May;4(3):255–261. doi: 10.1093/jac/4.3.255. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. Piperacillin, a new penicillin active against many bacteria resistant to other penicillins. Antimicrob Agents Chemother. 1978 Mar;13(3):358–367. doi: 10.1128/aac.13.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron J. A., Meyers B. R., Hirschman S. Z., Srulevitch E. Pharmacokinetics of piperacillin in patients with moderate renal failure and in patients undergoing hemodialysis. Antimicrob Agents Chemother. 1981 Feb;19(2):279–283. doi: 10.1128/aac.19.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup J. R., Benjamin D. R. Numerical integration simulation programs for the microcomputer. Ther Drug Monit. 1980;2(3):243–247. doi: 10.1097/00007691-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Latos D. L., Bryan C. S., Stone W. J. Carbenicillin therapy in patients with normal and impaired renal function. Clin Pharmacol Ther. 1975 Jun;17(6):692–700. doi: 10.1002/cpt1975176692. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Terry P. M. Susceptibility of gram-negative aerobic bacilli resistant to carbenicillin in a general hospital to piperacillin and ticarcillin. Antimicrob Agents Chemother. 1979 Jan;15(1):137–139. doi: 10.1128/aac.15.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers B. R., Hirschman S. Z., Strougo L., Srulevitch E. Comparative study of piperacillin, ticarcillin, and carbenicillin pharmacokinetics. Antimicrob Agents Chemother. 1980 Apr;17(4):608–611. doi: 10.1128/aac.17.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. F., Neu H. C. Pharmacokinetics of ticarcillin in patients with abnormal renal function. J Infect Dis. 1976 Jan;133(1):46–49. doi: 10.1093/infdis/133.1.46. [DOI] [PubMed] [Google Scholar]
- Prince A. S., Neu H. C. Use of piperacillin, a semisynthetic penicillin, in the therapy of acute exacerbations of pulmonary disease in patients with cystic fibrosis. J Pediatr. 1980 Jul;97(1):148–151. doi: 10.1016/s0022-3476(80)80157-0. [DOI] [PubMed] [Google Scholar]
- Sawchuk R. J., Zaske D. E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976 Apr;4(2):183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- Simon G. L., Snydman D. R., Tally F. P., Gorbach S. L. Clinical trial of piperacillin with acquisition of resistance by Pseudomonas and clinical relapse. Antimicrob Agents Chemother. 1980 Jul;18(1):167–170. doi: 10.1128/aac.18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. I., Russo M. E., Matsen J. M., Atkin-Thor E. Piperacillin pharmacokinetics in subjects with chronic renal failure. Antimicrob Agents Chemother. 1981 Mar;19(3):450–453. doi: 10.1128/aac.19.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandramaga T. B., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. Antimicrob Agents Chemother. 1978 Dec;14(6):829–837. doi: 10.1128/aac.14.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. Comparison of the activities of the new ureidopenicillins piperacillin, mezlocillin, azlocillin, and Bay k 4999 against gram-negative organisms. Antimicrob Agents Chemother. 1979 Aug;16(2):115–119. doi: 10.1128/aac.16.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston D. J., Murphy W., Young L. S., Hewitt W. L. Piperacillin therapy for serious bacterial infections. Am J Med. 1980 Aug;69(2):255–261. doi: 10.1016/0002-9343(80)90386-1. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Wang D., Young L. S., Martin W. J., Hewitt W. L. In vitro studies of piperacilin, a new semisynthetic penicillin. Antimicrob Agents Chemother. 1978 Jun;13(6):944–950. doi: 10.1128/aac.13.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]


