Abstract
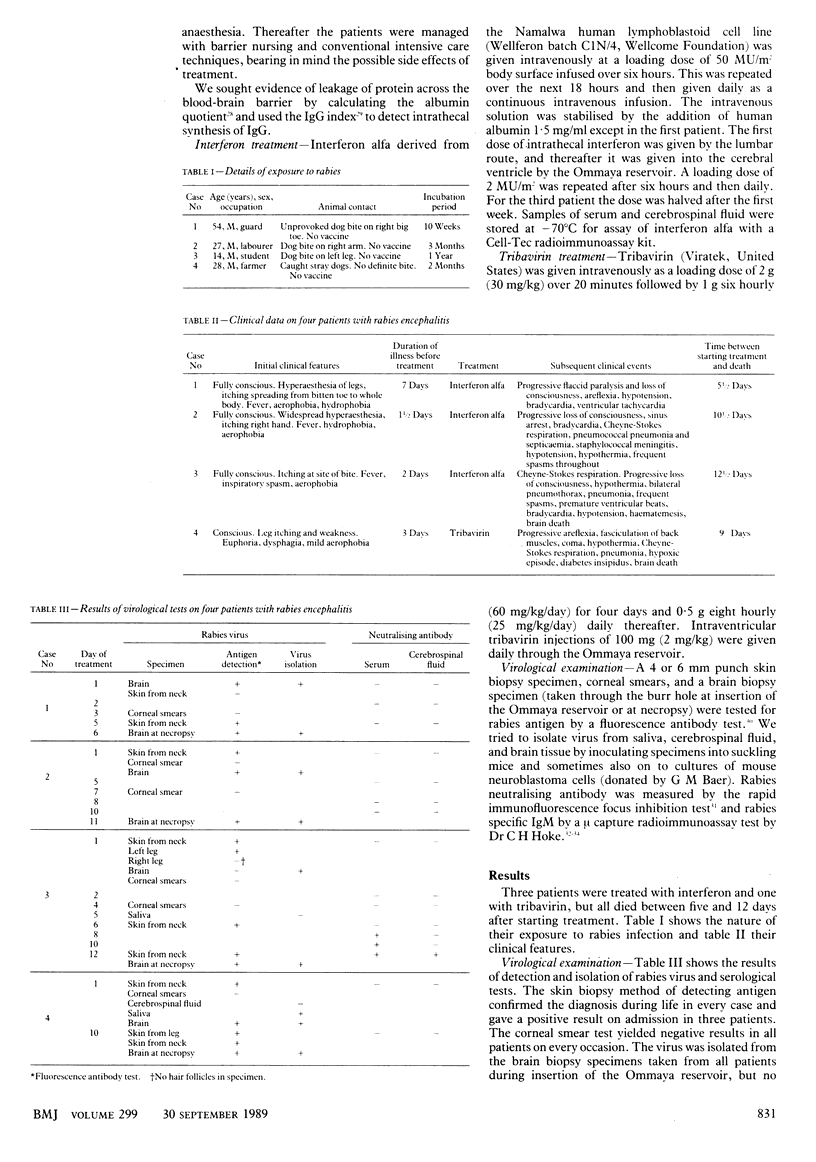
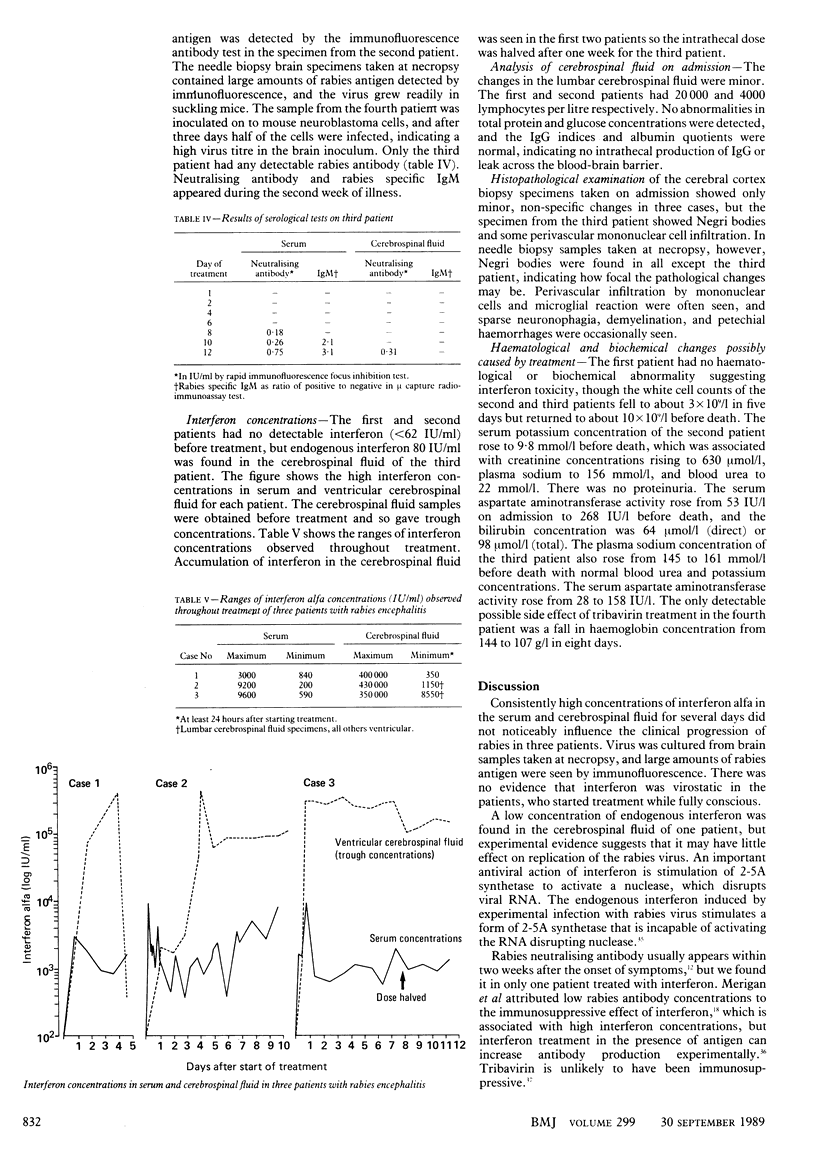
OBJECTIVE--To test the effect of interferon alfa and tribavirin (ribavirin) in patients with rabies encephalitis. DESIGN--An open trial of chemotherapy and intensive care in patients with early rabies. SETTING--The intensive care unit of a Bangkok hospital. PATIENTS--Four conscious men with clinical rabies encephalitis. INTERVENTIONS--Rapid virological diagnosis of rabies. Treatment with intravenous and intraventricular injections of high doses of lymphoblastoid interferon alfa in three patients and tribavirin in one patient. Intensive care was given throughout. MAIN OUTCOME MEASURES--Rabies infection confirmed by antigen detection and virus isolation. Rabies neutralising antibody and specific IgM sought in serum and cerebrospinal fluid. Interferon concentrations monitored before and during treatment in three patients. RESULTS--Interferon alfa treatment produced high concentrations in serum and cerebrospinal fluid. All four patients died after 5 1/2 to 12 1/2 days of treatment with no evidence of virostatic or clinically beneficial effects from either treatment. CONCLUSION--Interferon alfa treatment is not effective in rabies encephalitis. The use of tribavirin warrants further study, possibly combined with new therapeutic methods.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Nicholson K. G., Tauxe R. V., Winkler W. G. Human rabies in the United States, 1960 to 1979: epidemiology, diagnosis, and prevention. Ann Intern Med. 1984 May;100(5):728–735. doi: 10.7326/0003-4819-100-5-728. [DOI] [PubMed] [Google Scholar]
- Baer G. M., Shaddock J. H., Williams L. W. Prolonging morbidity in rabid dogs by intrathecal injection of attenuated rabies vaccine. Infect Immun. 1975 Jul;12(1):98–103. doi: 10.1128/iai.12.1.98-103.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt D. R., Hattwick M. A., Gerdsen R., Emmons R. W., Johnson H. N. Human rabies. Diagnosis, complications, and management. Am J Dis Child. 1974 Jun;127(6):862–869. doi: 10.1001/archpedi.1974.02110250088013. [DOI] [PubMed] [Google Scholar]
- Blenden D. C., Creech W., Torres-Anjel M. J. Use of immunofluorescence examination to detect rabies virus antigen in the skin of humans with clinical encephalitis. J Infect Dis. 1986 Oct;154(4):698–701. doi: 10.1093/infdis/154.4.698. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Ussery M. A. Antibody capture immunoassay detection of japanese encephalitis virus immunoglobulin m and g antibodies in cerebrospinal fluid. J Clin Microbiol. 1982 Dec;16(6):1034–1042. doi: 10.1128/jcm.16.6.1034-1042.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussereau F., Picard M., Blancou J., Sureau P. Treatment of rabies in mice and foxes with antiviral compounds. Acta Virol. 1988 Jan;32(1):33–49. [PubMed] [Google Scholar]
- Canonico P. G. Efficacy, toxicology and clinical applications of ribavirin against virulent RNA viral infections. Antiviral Res. 1985;Suppl 1:75–81. doi: 10.1016/s0166-3542(85)80011-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Human rabies--Texas. MMWR Morb Mortal Wkly Rep. 1984 Aug 24;33(33):469–470. [PubMed] [Google Scholar]
- Cohen S. L., Gardner S., Lanyi C., McDonald J. R., Rée H., Southorn P. A., Woodruff A. W. A case of rabies in man: some problems in diagnosis and management. Br Med J. 1976 May 1;1(6017):1041–1042. doi: 10.1136/bmj.1.6017.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C., Bubley G., Lucey D., Hussey S., Connor J. Ribavirin enters cerebrospinal fluid. Lancet. 1986 Jul 5;2(8497):45–46. doi: 10.1016/s0140-6736(86)92590-0. [DOI] [PubMed] [Google Scholar]
- DEPOUX R. VIRUS RABIQUE FIXE ET INTERF'ERON. C R Hebd Seances Acad Sci. 1965 Jan 4;260:354–356. [PubMed] [Google Scholar]
- Dean D. J., Abelseth M. K. Laboratory techniques in rabies: the fluorescent antibody test. Monogr Ser World Health Organ. 1973;(23):73–84. [PubMed] [Google Scholar]
- Emmons R. W., Leonard L. L., DeGenaro F., Jr, Protas E. S., Bazeley P. L., Giammona S. T., Sturckow K. A case of human rabies with prolonged survival. Intervirology. 1973;1(1):60–72. doi: 10.1159/000148833. [DOI] [PubMed] [Google Scholar]
- Fenje P., Postic B. Protection of rabbits against experimental rabies of poly I-poly C. Nature. 1970 Apr 11;226(5241):171–172. doi: 10.1038/226171a0. [DOI] [PubMed] [Google Scholar]
- Hattwick M. A., Weis T. T., Stechschulte C. J., Baer G. M., Gregg M. B. Recovery from rabies. A case report. Ann Intern Med. 1972 Jun;76(6):931–942. doi: 10.7326/0003-4819-76-6-931. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Pratt R. D., Elm J. L., Jr, Venkateshan C. N., Halstead S. B. Treatment of intracranial dengue virus infections in mice with a lipophilic derivative of ribavirin. Antimicrob Agents Chemother. 1983 Jul;24(1):134–136. doi: 10.1128/aac.24.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin O. L., Longstreth J. A., Hart C. C., Scavuzzo D., Kalman C. M., Connor J. D., Roberts R. B. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987 May;41(5):546–555. doi: 10.1038/clpt.1987.70. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Pollard J. D., Davis J. N. Human rabies encephalomyelitis. Br Med J. 1976 May 1;1(6017):1038–1040. doi: 10.1136/bmj.1.6017.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. B., King I. J., Webb P. A., Scribner C. L., Craven R. B., Johnson K. M., Elliott L. H., Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986 Jan 2;314(1):20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Baer G. M., Winkler W. G., Bernard K. W., Gibert C. G., Chany C., Veronesi R. Human leukocyte interferon administration to patients with symptomatic and suspected rabies. Ann Neurol. 1984 Jul;16(1):82–87. doi: 10.1002/ana.410160116. [DOI] [PubMed] [Google Scholar]
- Rohatiner A. Z., Balkwill F. R., Griffin D. B., Malpas J. S., Lister T. A. A phase I study of human lymphoblastoid interferon administered by continuous intravenous infusion. Cancer Chemother Pharmacol. 1982;9(2):97–102. doi: 10.1007/BF00265387. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Sullivan L., Summers R., Gregg M. B., Sikes R. K. A case of human rabies in Kansas: epidemiologic, clinical, and laboratory considerations. J Infect Dis. 1970 Oct;122(4):318–322. doi: 10.1093/infdis/122.4.318. [DOI] [PubMed] [Google Scholar]
- Sidwell R. W., Allen L. B., Khare G. P., Huffman J. H., Witkowski J. T., Simon L. N., Robins R. K. Effect of 1-beta-D-ribofuranosyl1-1,2,4-triazole-3-carboxamide (virazole, ICN 1229) on herpes and vaccinia keratitis and encephalitis in laboratory animals. Antimicrob Agents Chemother. 1973 Feb;3(2):242–246. doi: 10.1128/aac.3.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973 May;48(5):535–541. [PMC free article] [PubMed] [Google Scholar]
- Thompson E. J., Riches P. G., Kohn J. Antibody synthesis within the central nervous system: comparisons of CSF IgG indices and electrophoresis. J Clin Pathol. 1983 Mar;36(3):312–315. doi: 10.1136/jcp.36.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingpalapong M., Hoke C. H., Jr, Ward G. S., Burke D. S., Elwell M. R., Lohytyothin S., Saisombat S. Anti-rabies virus IgM in serum and cerebrospinal fluid from rabid dogs. Southeast Asian J Trop Med Public Health. 1986 Dec;17(4):550–557. [PubMed] [Google Scholar]
- Warrell D. A., Davidson N. M., Pope H. M., Bailie W. E., Lawrie J. H., Ormerod L. D., Kertesz A., Lewis P. Pathophysiologic studies in human rabies. Am J Med. 1976 Feb;60(2):180–190. doi: 10.1016/0002-9343(76)90427-7. [DOI] [PubMed] [Google Scholar]
- Warrell M. J., Looareesuwan S., Manatsathit S., White N. J., Phuapradit P., Vejjajiva A., Hoke C. H., Burke D. S., Warrell D. A. Rapid diagnosis of rabies and post-vaccinal encephalitides. Clin Exp Immunol. 1988 Feb;71(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Warrell M. J., Ward G. S., Elwell M. R., Tingpalapong M. An attempt to treat rabies encephalitis in monkeys with intrathecal live rabies virus RV 675. Brief report. Arch Virol. 1987;96(3-4):271–273. doi: 10.1007/BF01320967. [DOI] [PubMed] [Google Scholar]
- Webster W. A., Casey G. A., Charlton K. M., Sayson R. C., McLaughlin B., Noble M. A. A case of human rabies in western Canada. Can J Public Health. 1987 Nov-Dec;78(6):412–413. [PubMed] [Google Scholar]
- Weinmann E., Majer M., Hilfenhaus J. Intramuscular and/or intralumbar postexposure treatment of rabies virus-infected cynomolgus monkeys with human interferon. Infect Immun. 1979 Apr;24(1):24–31. doi: 10.1128/iai.24.1.24-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Postic B., Ho M., Koprowski H. Role of interferon induction in the protective activity of rabies vaccines. J Infect Dis. 1972 Oct;126(4):408–418. doi: 10.1093/infdis/126.4.408. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Shaw M., Silverman L. M., Eisenberg R. A., Wilson H. A., 3rd, Koffler D. Intrathecal IgG synthesis and blood-brain barrier impairment in patients with systemic lupus erythematosus and central nervous system dysfunction. Am J Med. 1983 May;74(5):837–844. doi: 10.1016/0002-9343(83)91075-6. [DOI] [PubMed] [Google Scholar]


