Abstract
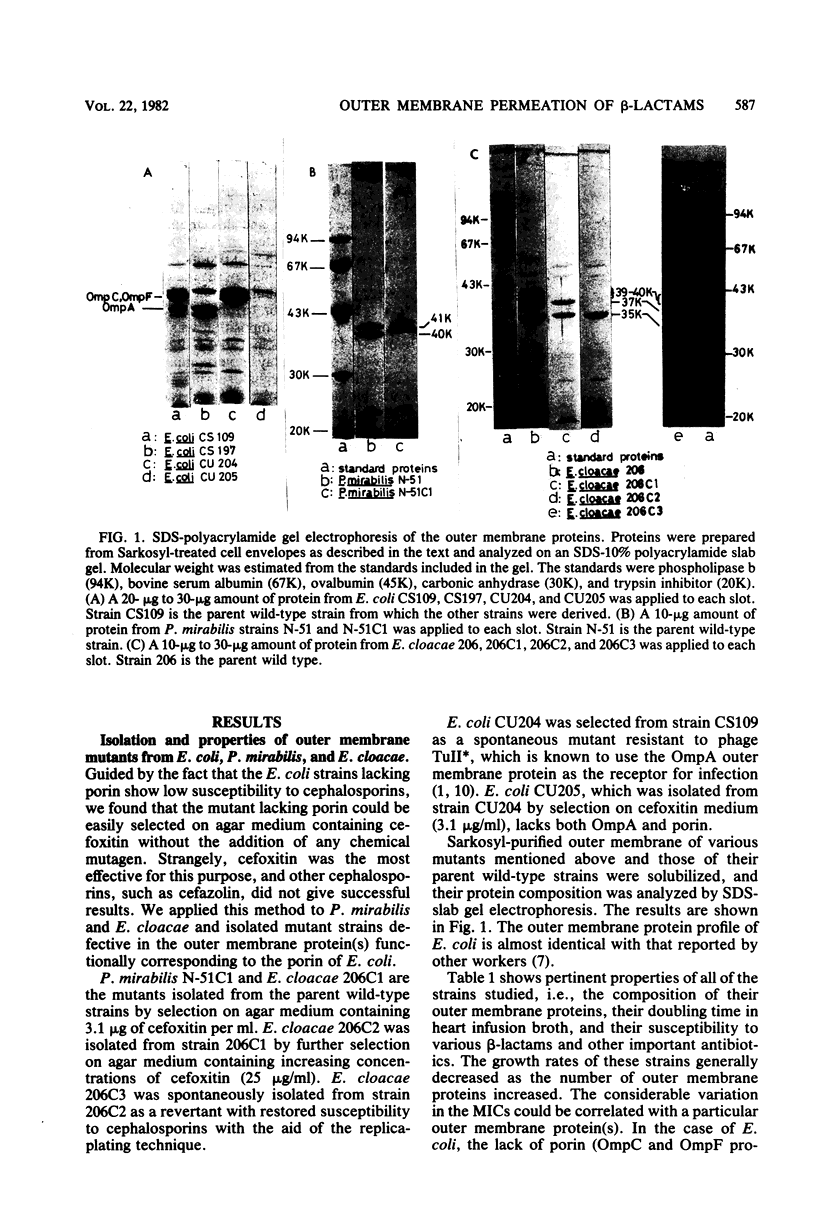
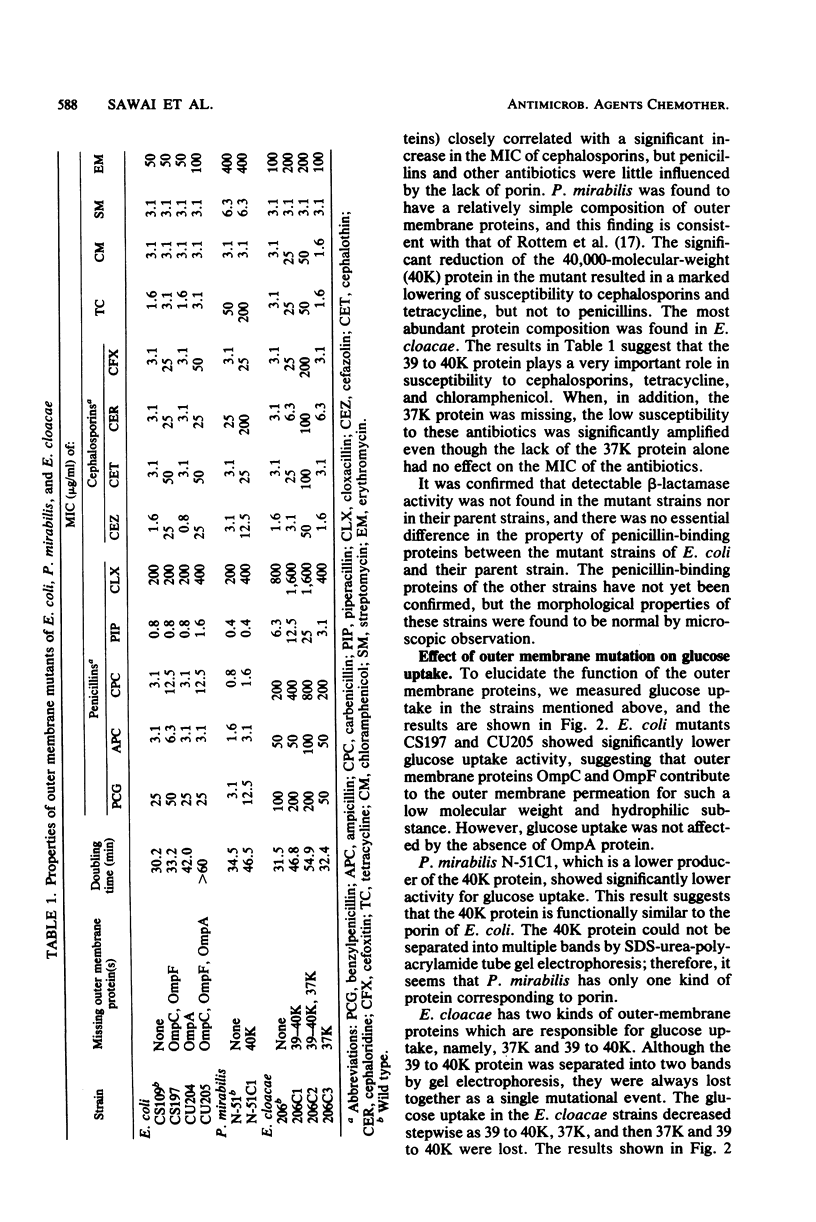
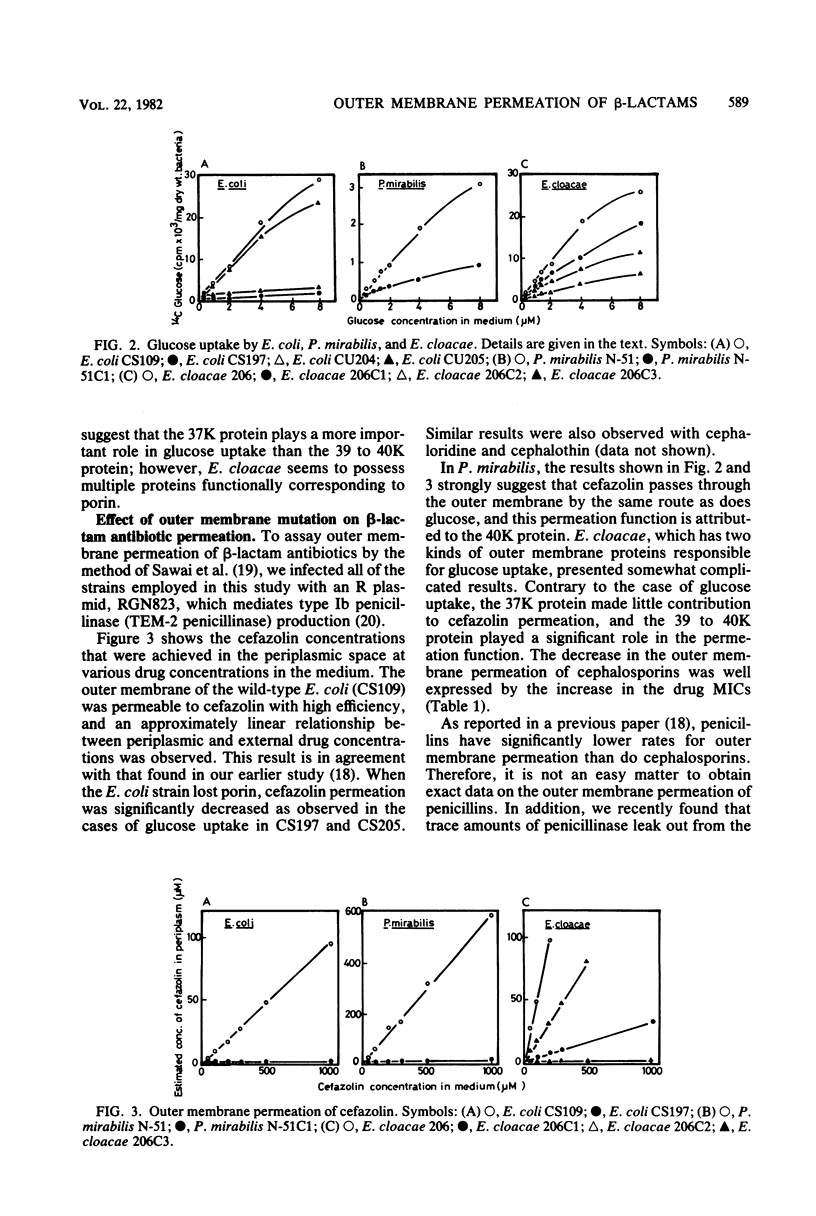
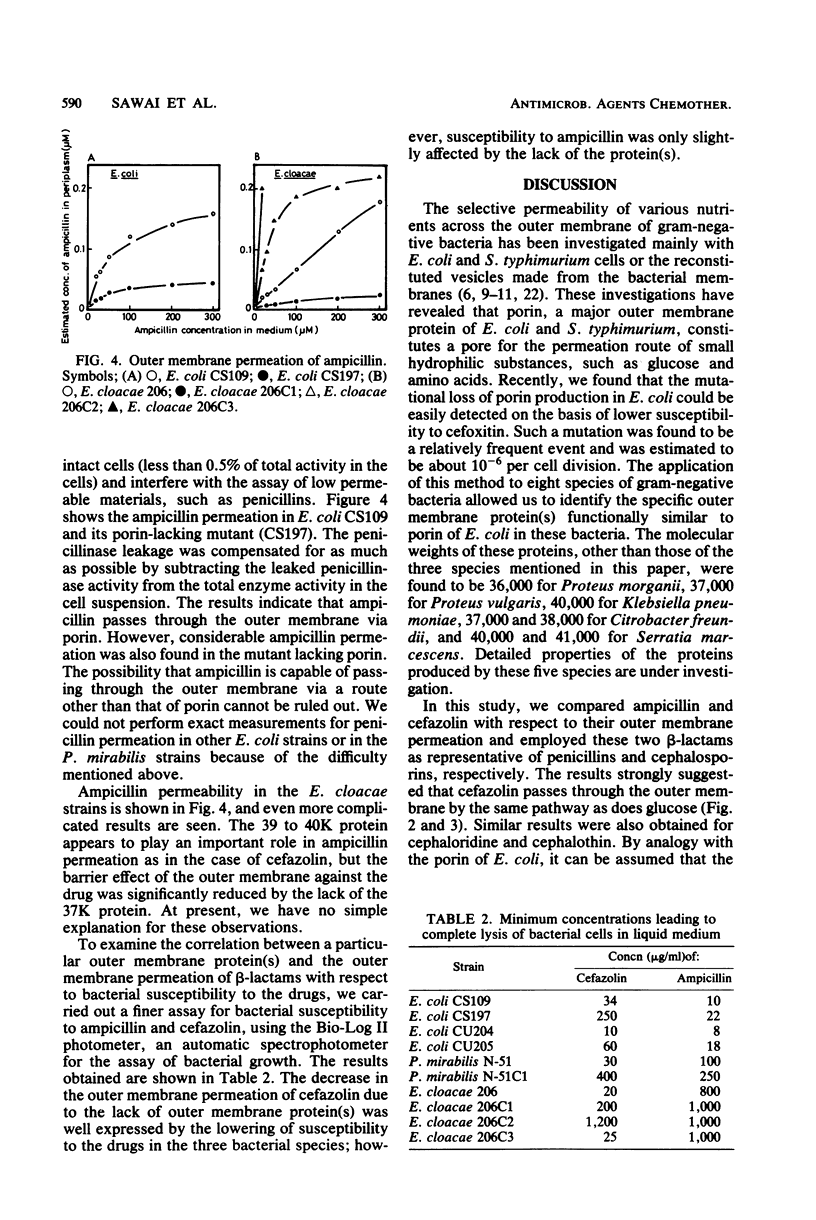
Mutant strains lacking outer membrane protein(s) were isolated from Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. The outer membrane protein(s) of P. mirabilis and E. cloacae corresponding to E. coli porin were identified on the basis of their function, namely, their ability to allow the permeation of glucose as demonstrated by [14C]glucose uptake by intact cells. P. mirabilis has only one outer membrane pore protein (molecular weight, 40,000), but E. cloacae has at least two such proteins (molecular weights, 37,000 and 39,000 to 40,000). When the bacteria lost these proteins or porin, the outer membrane permeation of cefazolin was found to be greatly reduced in these three species. Such a change in the outer membrane permeation closely correlated with a significant decrease in the bacterial susceptibility to cephalosporins, including cefoxitin. These results suggested that the main pathway for cephalosporin permeation is the pore made up of these proteins. The 39,000- to 40,000-molecular-weight protein in E. cloacae was also assumed to play an important role in the outer membrane permeation of tetracycline and chloramphenicol. On the other hand, the permeation route of penicillins was obscure. The susceptibility to penicillins, except in some cases, was little influenced by the absence of the proteins. Ampicillin was found to pass through the outer membrane via the same route as the cephalosporins, but the possibility that ampicillin and other penicillins possess another unknown route for outer membrane permeation could not be ruled out.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Phage T6--colicin K receptor and nucleoside transport in Escherichia coli. FEBS Lett. 1976 Nov;70(1):109–112. doi: 10.1016/0014-5793(76)80737-5. [DOI] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. The tsx protein of Escherichia coli can act as a pore for amino acids. J Bacteriol. 1981 Sep;147(3):1113–1116. doi: 10.1128/jb.147.3.1113-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Diffusion of solutes through channels produced by phage lambda receptor protein of Escherichia coli: inhibition by higher oligosaccharides of maltose series. Biochem Biophys Res Commun. 1980 Mar 13;93(1):166–171. doi: 10.1016/s0006-291x(80)80261-0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Markowitz O., Hasin M., Razin S. Outer membrane proteins of smooth and rough strains of Proteus mirabilis. Eur J Biochem. 1979 Jun;97(1):141–146. doi: 10.1111/j.1432-1033.1979.tb13095.x. [DOI] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Tamura A., Yamagishi S. The bacterial outer-membrane permeability of beta-lactam antibiotics. J Antibiot (Tokyo) 1979 Jan;32(1):59–65. doi: 10.7164/antibiotics.32.59. [DOI] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Yamagishi S. A method for measuring the outer membrane-permeability of beta-lactam antibiotics in gram-negative bacteria. J Antibiot (Tokyo) 1977 Dec;30(12):1134–1136. doi: 10.7164/antibiotics.30.1134. [DOI] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Nakae T. The outer membrane permeability of Gram-negative bacteria: determination of permeability rate in reconstituted vesicle membranes. FEBS Lett. 1979 Oct 1;106(1):85–88. doi: 10.1016/0014-5793(79)80700-0. [DOI] [PubMed] [Google Scholar]



