Abstract
Several lines of evidence suggest that extracellular signal-regulated kinase1/2 (ERK1/2) and dopaminergic system is involved in learning and memory. However, it remains to be determined if the dopaminergic system and ERK1/2 pathway contribute to cognitive function in the prefrontal cortex (PFC). The amount of phosphorylated ERK1/2 was increased in the PFC immediately after exposure to novel objects in the training session of the novel object recognition test. An inhibitor of ERK kinase impaired long-term recognition memory 24 h after the training although short-term memory tested 1 h after the training was not affected by the treatment. The dopamine D1 receptor agonist increased ERK1/2 phosphorylation in the PFC in vivo as well as in cortical neurons in vitro. Microinjection of the dopamine D1 receptor antagonist into the PFC impaired long-term recognition memory whereas the D2 receptor antagonist had no effect. Immunohistochemistry revealed that exposure to novel objects resulted in an increase in c-Fos expression in the PFC. Microinjection of the protein synthesis inhibitor anisomycin into the PFC impaired the long-term recognition memory. These results suggest that the activation of ERK1/2 following the stimulation of dopamine D1 receptors is necessary for the protein synthesis-dependent long-term retention of recognition memory in the PFC.
Recognition memory is a fundamental facet of ability to remember and an integral component of the class of memory lost in amnesia (Aggleton and Brown 1999). The ability to discriminate familiar from novel stimuli is supported by this form of memory and is widely used as an assay in animals (Brown and Aggleton 2001). Recent work highlights a major role in recognition memory for the perirhinal cortex because lesions or transient inactivation of perirhinal cortex consistently disrupt performance on familiarity discrimination tasks with object in both primates and rats (Aggleton and Brown 1999; Murray and Richmond 2001; Winters and Bussey 2005b). Research on the perirhinal cortex has demonstrated that dysfunction of glutamate receptors impairs recognition memory (Winters and Bussey 2005a; Barker et al. 2006). In addition to the perirhinal cortex, it has been reported that the prefrontal cortex (PFC) plays a critical role in recognition memory (Meunier et al. 1997; Ragozzino et al. 2002), and microinjection of N-methyl-D-aspartate (NMDA) antagonist into the PFC induces memory impairment (Akirav and Maroun 2006).
Dopamine transmission in the PFC functionally regulates the higher motor functions of behavior, motivation, and cognition (Egan and Weinberger 1997; Lewis et al. 1998; Yang et al. 1999). In the PFC of rodents and monkeys, both the amount of receptor mRNA and the number of receptor-binding sites are significantly greater for the dopamine D1 receptor than for the other dopamine receptor subtypes (Farde et al. 1987; Lidow et al. 1991; Goldman-Rakic et al. 1992; Gaspar et al. 1995). Dopamine D1 receptors may thus play an important role in regulating the functions of the PFC. For instance, a disruption of dopamine transmission in the PFC caused by infusions of dopamine D1 receptor antagonists or by excitotoxic lesions impaired the working memory in nonhuman primates (Sawaguchi and Goldman-Rakic 1991, 1994). There are a few studies that have investigated the role of dopamine D1 receptors in the recognition memory. Systemic administration of the dopamine D1 antagonist reduced preference for novel objects (Besheer et al. 1999), whereas dopamine D1 receptor agonist potentiated memory retrieval in recognition memory in rats (Hotte et al. 2005). However, the mechanisms by which the dopaminergic system regulates recognition memory in the PFC remain unclear.
The storage of long-term memory, or memory consolidation, requires new mRNA and protein synthesis whereas short-term memory is insensitive to inhibitors of transcription and translation (Davis and Squire 1984; McGaugh 2000). It is well established that extracellular signal-regulated kinase1/2 (ERK1/2), a member of the mitogen-activated protein kinase (MAPK) family, plays an important role in transcriptional regulation in many cell types, including neurons (Treisman 1996). The ERK signaling pathway is a highly conserved kinase cascade linking transmembrane receptors to downstream effector mechanisms (Chang and Karin 2001; Pearson et al. 2001). In neurons, ERK1/2 signaling is activated by stimuli associated with synaptic activity and plasticity, most notably calcium influx and neurotrophins (McAllister et al. 1999; West et al. 2001; Tyler et al. 2002). It has been demonstrated that ERK1/2 is involved in long-term memory, but not memory acquisition or short-term memory, in the insular cortex, hippocampus (Blum et al. 1999), amygdala (Schafe et al. 2000), and entorhinal cortex (Hebert and Dash 2002). These results suggest that an increase in the level of phosphorylated ERK1/2 can be used as a correlate for long-term memory storage.
We have previously shown that the ERK1/2 plays a critical role in memory functions under physiological and pathological conditions (Mizoguchi et al. 2004; Kamei et al. 2006; Nagai et al. 2006). Especially, ERK1/2 signaling pathway linked to dopamine D1 receptors (Valjent et al. 2000; Zanassi et al. 2001) is involved in methamphetamine (METH)-associated contextual memory in rats (Mizoguchi et al. 2004). Moreover, we have demonstrated that repeated METH treatment in mice induces cognitive impairment in a novel object recognition test, which is accompanied by a dysfunction of the ERK1/2 pathway in the PFC (Kamei et al. 2006). Taken together, these results raise the possibility that the dopamine D1 receptor-ERK1/2 signaling pathway in the PFC is critically involved in molecular adaptations that are necessary for long-term behavioral and neuronal plasticity. In this study, we investigated the role of the dopamine D1 receptor-ERK1/2 signaling pathway in the PFC for recognition memory in mice.
Results
Effect of a MEK inhibitor on the novelty-induced ERK1/2 phosphorylation in the PFC
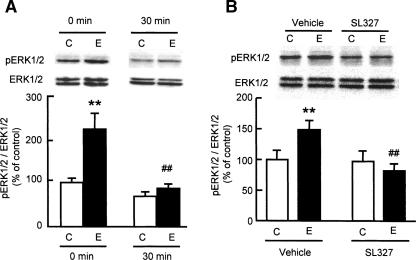
We first examined the changes in levels of phosphorylated ERK1/2 in the PFC of mice following exposure to novel objects by Western blotting. A significant increase was observed immediately after a 10-min exposure (F(3,19) = 12.151, P < 0.01 by ANOVA, and P < 0.01 by post-hoc comparison; Fig. 1A), followed by a return to control levels within 30 min (Fig. 1A). On the other hand, no significant changes were observed in the hippocampus after exposure to the novel objects (data not shown). There were no differences in total ERK1/2 levels between the control and exposed groups in any region of the brain examined.
Figure 1.
Effect of a MEK inhibitor SL327 on novelty-induced ERK1/2 phosphorylation in the PFC. (A) Changes in ERK1/2 phosphorylation in the PFC of mice exposed to novel objects. Mice were killed immediately (0 min) or 30 min after the exposure (n = 5 for control [0 min] group; n = 6 for other groups). (B) Effect of SL327 on novelty-induced ERK1/2 phosphorylation in the PFC. SL327 (50 mg/kg, i.p.) was injected 60 min before the exposure to novel objects, and the mice were decapitated immediately after the exposure (n = 3 for vehicle-treated control group; n = 8 for vehicle-treated exposure group; n = 5 for SL327-treated control group; n = 8 for SL327-treated exposure group). C, mice were not exposed to novel objects. E, mice were exposed to novel objects. Values represent the mean ± SE. ANOVA analysis: F(3,19) = 12.151, P < 0.01 for A; F(3,20) = 3.243, P < 0.05 for B. **P < 0.01 compared to corresponding control group. ##P < 0.01 compared to corresponding exposure group.
SL327 is a specific ERK kinase (MEK) inhibitor that penetrates the blood-brain barrier. It is reported that a peripheral injection of SL327 dose-dependently (0–100 mg/kg) reduced levels of phosphorylated ERK1/2 in rat hippocampus and frontal cortex, the effect reaching a plateau at a dose of 50 mg/kg (Selcher et al. 1999). The novelty-induced ERK1/2 phosphorylation in the PFC was significantly abolished by SL327 (50 mg/kg, i.p.) treatment (F(3,20) = 3.243, P < 0.05 by ANOVA, and P < 0.01 by post-hoc comparison; Fig. 1B). There was no concurrent decrease in total ERK1/2 levels with SL327 (Fig. 1B).
Effect of a MEK inhibitor on performance in the novel object recognition test
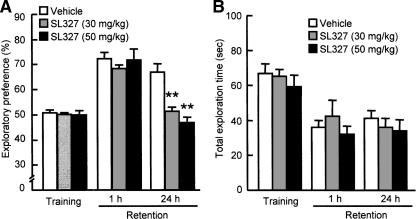
Next, we tested whether novelty-induced activation of ERK1/2 in the PFC is associated with recognition memory in the novel object recognition test. To this end, SL327 (30 or 50 mg/kg, i.p.) was injected 1 h before the training session. During the training session, SL327-treated and vehicle-treated mice spent equal amounts of time exploring either of the two objects (Fig. 2A), and thus there was no biased exploratory preference in either group of animals. Moreover, SL327 had no effect on total exploration time for the objects (Fig. 2B), suggesting that the MEK inhibitor has no effect on motivation, curiosity, or motor function.
Figure 2.
Effect of a MEK inhibitor SL327 on performance in the novel object recognition test. (A) Exploratory preference. (B) Total exploration time. SL327 (30 or 50 mg/kg, i.p.) was injected 60 min before the training session, and the retention session was carried out either 1 or 24 h after the training session. Values represent the mean ± SE (n = 6). ANOVA analysis: F(2,15) = 26.844, P < 0.01 in exploratory preference of retention session 24 h after the training. **P < 0.01 compared to corresponding vehicle-treated group.
The retention session was carried out 1 or 24 h after the training session. In the 1-h retention session, SL327-treated mice exhibited the same level of exploratory preference for a novel object as did vehicle-treated mice (Fig. 2A). However, when retention performance was tested 24 h after the training session, the level of exploratory preference for the novel object in the SL327-treated mice was significantly decreased compared to that in the vehicle-treated mice (F(2,15) = 26.844, P < 0.01 by ANOVA, P < 0.01 by post-hoc comparison; Fig. 2A). The total exploration time did not differ among the three groups in the retention session. These results suggest that inhibition of ERK1/2 by SL327 treatment results in an impairment of long-term memory retention without affecting memory acquisition (learning) and short-term memory retention.
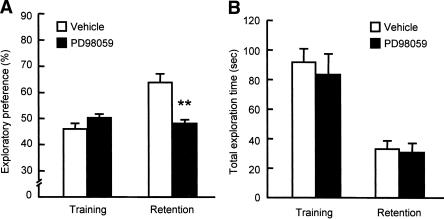
These results are consistent with our previous findings that microinjection of PD98059, a selective MEK inhibitor, into the PFC before the training session impaired the object recognition memory when tested 24 h after the training (Kamei et al. 2006). In the present study, we also tested the effect of PD98059 microinjected into the hippocampus of mice before the training session. In the training session, bilateral microinjections of PD98059 into the hippocampus (2 μg/side) did not affect the exploratory preference or the total exploration time (Fig. 3A). However, when memory retention was tested 24 h after the training session, the level of exploratory preference for a novel object in the PD98059-treated mice was significantly decreased compared to that in the vehicle-treated mice (F(3,28) = 11.736, P < 0.01 by ANOVA, P < 0.01 by post-hoc comparison; Fig. 3A), without affecting total exploration time in the retention session (Fig. 3B).
Figure 3.
Effects of bilateral intra-hippocampal injections of a MEK inhibitor PD98059 on performance in the novel object recognition test. (A) Exploratory preference. (B) Total exploration time. PD98059 (2 μg/1.0 μL/side) was microinjected bilaterally into the hippocampus of mice 20 min before the training session. The retention session was carried out 24 h after the training session. Values indicate the mean ± SE (n = 8). ANOVA analysis: F(3,28) = 11.736, P < 0.01 for A. **P < 0.01 compared to corresponding vehicle-treated group.
Effects of dopamine D1 and D2 receptor antagonists on recognition memory
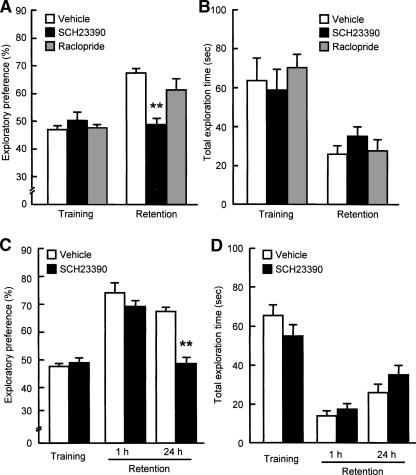
To explore the role dopamine receptors in the PFC play in recognition memory, we examined the effects of bilateral microinjections of a selective dopamine D1 receptor antagonist, SCH23390 (1 μg/side), or a dopamine D2 receptor antagonist, raclopride (5 μg/side), into the PFC on performance in the novel object recognition test. For the doses of SCH23390 and raclopride, we referred to the studies by Runyan and Dash (2004) and Greba et al. (2001), respectively. Bilateral microinjections of SCH23390 into the PFC had no effect on the exploratory preference or the total exploration time in the training session (Fig. 4A,B, respectively). However, when memory retention was tested 24 h after the training session, SCH23390-treated mice showed a significant decrease in the exploratory preference for a novel object compared to the vehicle-treated mice (F(2,22) = 9.915, P < 0.01 by ANOVA, P < 0.01 by post-hoc comparison; Fig. 4A). In contrast, bilateral microinjections of raclopride into the PFC had no effect on exploratory preference in the training and retention sessions (Fig. 4A). These results suggest that dopamine D1 receptors in the PFC play a role in object recognition memory.
Figure 4.
Effects of bilateral intra-PFC injections of dopamine D1 or D2 receptor antagonist on performance in the novel object recognition test. (A,C) Exploratory preference. (B,D) Total exploration time. (A,B) SCH23390 (1 μg/0.5 μL/side) or raclopride (5 μg/0.5 μL/side) was microinjected bilaterally into the PFC of mice 15 min before the training session. The retention session was carried out 24 h (n = 7 for vehicle-treated group, n = 9 for SCH23390-treated group, n = 9 for raclopride-treated group) after the training session. (C,D) SCH23390 (1 μg/0.5 μL/side) was bilaterally microinjected into the PFC of mice 15 min before the training session. The retention session was carried out either 1 h (n = 9 for vehicle-treated group, n = 11 for SCH23390-treated group) or 24 h (n = 7 for vehicle-treated group, n = 9 for SCH23390-treated group) after the training session. Values indicate the mean ± SE. ANOVA analysis: F(2,22) = 9.915, P < 0.01 for A; F(3,32) = 15.519, P < 0.01 for C. **P < 0.01 compared to corresponding vehicle-treated group.
To further characterize the memory component(s) that was inhibited by SCH23390, we used a different batch of mice. When the retention was tested 1 h after the training, SCH23390 had no effect on the exploratory preference for a novel object or the total exploration time (Fig. 4C,D). However, the exploratory preference for a novel object disappeared in the SCH23390-treated mice, when the retention session was carried out 24 h after the training session (F(3,32) = 15.519, P < 0.01 by ANOVA, P < 0.01 by post-hoc comparison; Fig. 4C). These results suggest that a blockade of dopamine D1 receptors in the PFC leads to a deficit of long-term retention of object recognition memory without affecting memory acquisition or short-term memory retention.
Effect of a dopamine D1 receptor agonist on ERK1/2 phosphorylation in the PFC
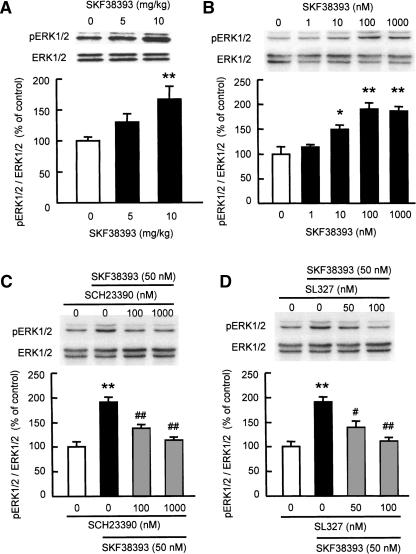
Next, we investigated whether stimulating dopamine D1 receptors promotes the phosphorylation of ERK1/2 in the PFC in vivo. We determined the levels of phosphorylated ERK1/2 in the PFC of mice following treatment with a selective dopamine D1 receptor agonist, SKF38393. SKF38393 caused a robust dose-dependent increase in phosphorylated ERK1/2 levels in the PFC of mice, with a significant change at a dose of 10 mg/kg (F(2,24) = 5.188, P < 0.05 by ANOVA, and P < 0.01 by post-hoc comparison; Fig. 5A).
Figure 5.
Dopamine D1 receptor agonist-induced ERK1/2 phosphorylation in the PFC of mice in vivo (A) and in cortical neurons in vitro (B–D). (A) Mice were killed 30 min after the treatment with SKF38393 (5 or 10 mg/kg, i.p.) (n = 9). (B) Primary cultured cortical neurons (8–10 DIV) were treated with increasing concentrations of SKF38393 (1–1000 nM) for 30 min (n = 6). (C) Effect of a D1 receptor antagonist SCH23390 on SKF38393-induced ERK1/2 phosphorylation in cultured neurons. (D) Effect of a MEK inhibitor SL327 on SKF38393-induced ERK1/2 phosphorylation in cultured neurons. SCH23390 and SL327 were added to the culture 15 min prior to SKF38393 (50 nM) treatment (n = 8 for control and SKF38393, n = 7 for SCH23390 and SL327). Values indicate the mean ± SE. ANOVA analysis: F(2,24) = 5.188, P < 0.05 for A; F(4,25) = 14.024, P < 0.01 for B; F(3,26) = 20.277, P < 0.01 for C; F(3,26) = 15.748, P < 0.01 for D. **P < 0.01 compared to vehicle-treated group. #P < 0.05, ##P < 0.01 compared to SKF38393-treated group.
To confirm that the activation of dopamine D1 receptors induces the phosphorylation of ERK1/2 in cortical neurons, we stimulated primary cultured cortical neurons with SKF38393. The addition of SKF38393 to the culture medium resulted in a concentration-dependent increase in phosphorylated ERK1/2 levels (F(4,25) = 14.024, P < 0.01; Fig. 5B). The SKF38393-induced phosphorylation of ERK1/2 was dose-dependently and completely blocked by pretreatment with a selective dopamine D1 antagonist, SCH23390 (F(3,26) = 20.277, P < 0.01 by ANOVA, and P < 0.01 by post-hoc comparison; Fig. 5C) or MEK inhibitor, SL327 (F(3,26) = 15.748, P < 0.01 by ANOVA, and P < 0.01 by post-hoc comparison; Fig. 5D). Taken together, these results suggest that stimulating dopamine D1 receptors in the PFC directly activates ERK1/2 via MEK.
Novelty-induced c-Fos expression in the PFC and the effect of a protein synthesis inhibitor on recognition memory
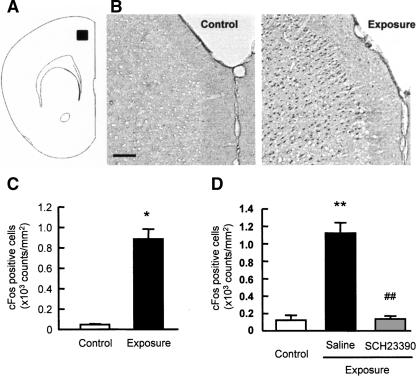
Molecular accounts of memory suggest that memories are changed from a labile to a more fixed state via a protein synthesis-dependent consolidation process (Davis and Squire 1984; Abel and Lattal 2001; Nader 2003). ERK1/2 activates downstream transcription factors, ets-like gene-1 (Elk-1) and cAMP response binding protein (CREB), which can then lead to transcription of immediate-early genes (IEGs), including c-Fos (Janknecht et al. 1993; Mayr et al. 2001). Therefore, we examined the changes in c-Fos expression levels in the PFC following exposure to the novel objects (Fig. 6). A marked increase in the numbers of c-Fos-positive cells was observed in the PFC of mice exposed to the novel objects (P < 0.05; Fig. 6B,C), and the novelty-induced increase in the numbers of c-Fos-positive cells was completely blocked by pretreatment with a selective dopamine D1 antagonist SCH23390 (F(2,13) = 68.151, P < 0.01 by ANOVA, P < 0.01 by post-hoc comparison; Fig. 6D).
Figure 6.
Changes in c-Fos expression in the PFC of mice exposed to novel objects. Mice were killed 2 h after a 10-min exposure to novel objects (Exposure). Control mice were not exposed to novel objects (Control). (A) Diagrammatic representation of the area examined for c-Fos immunostaining. (B) Representative photomicrographs of c-Fos immunostaining. Scale bar, 100 μm. (C) Quantification of the changes in the number of c-Fos-positive cells in the PFC (n = 4). (D) Effects of SCH23390 on c-Fos expression in the PFC of mice exposed to novel objects. Saline or SCH23390 (0.05 mg/kg, i.p.) was administered 30 min before training session (n = 5 for control group, n = 4 for saline-treated exposure group, n = 7 for SCH23390-treated exposure group). Values represent the mean ± SE. ANOVA analysis: F(2,13) = 68.151, P < 0.01 for D. *P < 0.05 and **P < 0.01 compared to control group. ##P < 0.01 compared to saline-treated exposure group.
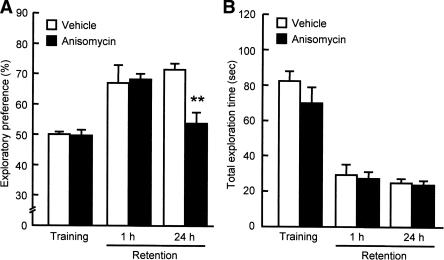
To investigate whether protein synthesis is necessary for consolidation of object recognition memory, anisomycin (50 μg/side) was microinjected bilaterally into the PFC before the training session. In the training session, pretreatment with anisomycin did not affect the exploratory preference for the objects or the total exploration time (Fig. 7A,B, respectively). Moreover, the anisomycin-treated mice showed the same level of exploratory preference for a novel object as did the vehicle-treated animals in the retention session which was carried out 1 h after the training session (Fig. 7A). However, when memory retention was tested 24 h after the training session, the level of exploratory preference for a novel object in the anisomycin-treated mice was significantly decreased compared to that in the vehicle-treated mice (F(3,34) = 5.591, P < 0.01 by ANOVA, P < 0.01 by post-hoc comparison; Fig. 7A). These results suggest that de novo protein synthesis is involved in long-term, but not short-term, recognition memory retention.
Figure 7.
Effects of bilateral intra-PFC injections of a protein synthesis inhibitor anisomycin on performance in the novel object recognition test. (A) Exploratory preference. (B) Total exploration time. Anisomycin (50 μg/0.5 μL/side) was microinjected bilaterally into the PFC of mice 30 min before the training session. The retention session was carried out either 1 h (n = 8 for vehicle-treated group, n = 7 for anisomycin-treated group) or 24 h (n = 12 for vehicle-treated group, n = 11 for anisomycin-treated group) after the training session. Values indicate the mean ± SE. ANOVA analysis: F(3,34) = 5.591, P < 0.01 for A. **P < 0.01 compared to vehicle-treated group.
Discussion
In the course of studying the mechanism underlying the enduring memory impairment induced by repeated METH treatment, we observed that ERK1/2 in the PFC was hyperphosphorylated when mice were exposed to novel objects and that pharmacological inhibition of ERK1/2 by the microinjection of PD98059, a MEK inhibitor, into the PFC resulted in cognitive impairment (Kamei et al. 2006). Consistent with these observations, we demonstrated in the present study that exposure of mice to novel objects caused a transient increase in phosphorylated ERK1/2 levels and that the inhibition by SL327 of the novelty-induced transient activation of ERK1/2 in the PFC was associated with an impairment of long-term retention of object recognition memory although short-term memory was not affected by the inhibitor. These results are consistent with several other reports showing that activation of the ERK1/2 signaling cascade is necessary for consolidation of different forms of long-term memory (Brambilla et al. 1997; Atkins et al. 1998; Berman et al. 1998; Blum et al. 1999; Selcher et al. 1999; Walz et al. 1999, 2000; Kelleher et al. 2004). Accordingly, it is suggested that activation of ERK1/2 in the PFC is necessary for long-term retention, but not the acquisition (learning) or short-term retention, of object recognition memory.
It has been reported that phosphorylation of ERK1/2 is increased in the dentate gyrus of the hippocampus and entorhinal cortex of rats exposed to a novel object (Kelly et al. 2003). Although we failed to detect significant changes in the level of phosphorylated ERK1/2 in the hippocampus immediately after the exposure to novel objects in this study, microinjections of the MEK inhibitor PD98059 into the hippocampus impaired recognition memory. The reason for the discrepancy between our results and those of Kelly et al. (2003) is not clear, but may be due to the differences in species used (mouse vs. rat, respectively) or brain regions measured in each experiment (whole vs. subfield, respectively). Alternatively, our results may be supported by recent reports that cortical regions have a more critical role in the recognition of novel objects than does the hippocampus (Mumby 2001; Gilbert and Kesner 2003).
ERK1/2-linked signaling pathways are stimulated by receptor tyrosine kinases (Cobb and Goldsmith 1995) and G protein-coupled receptors (Post and Brown 1996). It is known that ERK1/2 is critically linked to dopamine D1 receptors which couple with Gs protein (Valjent et al. 2000; Zanassi et al. 2001). Several second messengers could be responsible for the link between dopamine D1 receptors and ERK1/2. Namely, cAMP, the first second messenger to be recognized as a modulator of ERK1/2’s phosphorylation, induces the phosphorylation of ERK1/2 via the small Ras-related G protein Rap1, which is activated by protein kinase A and interacts with the B Raf isoform (Vossler et al. 1997; Zanassi et al. 2001; Belcheva and Coscia 2002). In the present study, we found that a dopamine D1 receptor agonist promoted phosphorylation of ERK1/2 in the PFC of mice in vivo and in primary cultured cortical neurons in vitro. The results are consistent with previous reports that stimulating dopamine D1 receptors activates ERK1/2 signaling (Valjent et al. 2000, 2004; Chen et al. 2004).
Dopamine plays a critical role in cognitive function. For instance, the amount of dopamine released in the PFC increases in a phasic manner during both the acquisition and retrieval phases of the delayed response task, and the release is negatively correlated with the number of errors committed during memory retrieval (Phillips et al. 2004). Targeted deletion of dopamine D1 receptors impairs spatial learning without visual or motor impairment (El-Ghundi et al. 1999). Dopamine D1 receptor antagonist SCH23390, but not dopamine D2/D3 receptor antagonist eticlopride, blocks novelty-induced place preference (Besheer et al. 1999). Infusions of the dopamine D1 receptor antagonist SCH23390 or SCH39166 into the PFC of monkeys (Sawaguchi and Goldman-Rakic 1991, 1994) or rats (Seamans et al. 1998) produce a delay-related impairment in spatial working memory performance, whereas comparable infusions of dopamine D2 receptor antagonists show no effect. In the present study, we demonstrated that microinjection of a dopamine D1, but not D2, antagonist into the PFC impaired recognition memory 24 h after the training, without affecting short-term memory tested 1 h after the training, which is consistent with a previous observation that systemic administration of SCH23390 or eticlopride has no effect on recognition memory 1 h after the training (Besheer et al. 1999). Therefore, it is suggested that stimulating dopamine D1 receptors in the PFC is necessary for the long-term retention of recognition memory.
Novelty-induced, dopamine D1 receptor-mediated c-Fos expression was observed in the PFC of mice, and microinjection of the translational inhibitor anisomycin into the PFC impaired memory retention 24 h after the training in the novel object recognition test without affecting short-term memory tested 1 h after the training. Molecular mechanisms of recognition memory and protein synthesis in the PFC remain to be determined. Activation of the ERK1/2 pathway leads to the phosphorylation of transcriptional regulators such as CREB and Elk-1, and thus gene expression, suggesting a critical role for the ERK signaling pathway in synaptic and neuronal plasticity (Valjent et al. 2001; Miller and Marshall 2005). Recently, Kelleher et al. (2004) have demonstrated that inhibition of ERK1/2 blocks neuronal activity-induced translation as well as phosphorylation of the translation factors eIF4E and 4EBP1, and ribosomal protein S6. Taken together, these finding suggest that the novelty-induced stimulation of dopamine D1 receptors induces protein synthesis through the ERK1/2 signaling pathway in the PFC, which is involved in the consolidation of a labile recognition memory to a more fixed one in mice.
Whereas we demonstrated that activation of ERK1/2 following the stimulation of dopamine D1 receptors is necessary for the protein synthesis-dependent long-term retention of recognition memory, other studies have shown the involvement of NMDA receptors. For instance, microinjection of an NMDA receptor antagonist, D,L-2-amino-5-phosphonovaleric acid, into the ventromedial PFC results in an impairment of long-term, but not short-term, recognition memory (Akirav and Maroun 2006). Since NMDA receptor stimulation is known to activate ERK1/2 signaling (Chandler et al. 2001), the mutual regulation by dopamine D1 and NMDA receptors of ERK1/2 signaling in the PFC may play a role in long-term recognition memory.
The standard model of memory consolidation posits that memory is initially stored in the hippocampus and overtime is slowly transferred to the neocortex (McClelland et al. 1995). However, recent experimental findings indicate that, in addition to the hippocampus, long-term memory is stored in the neocortex at the time of training (Runyan et al. 2004). Our findings suggest that ERK-mediated synaptic plasticity in the hippocampus and PFC is required for memory storage, and are consistent with a theoretical model (the C theory) proposed by Dash et al. (2004) that long-term memory storage takes place both in the hippocampus and in the neocortex at the time of training.
In conclusion, we demonstrated that phosphorylation of ERK1/2 was transiently increased in the PFC immediately after exposure to novel objects in the training session of the novel object recognition test. Inhibition of ERK1/2 phosphorylation impaired long-term, but not short-term, recognition memory. Dopamine D1 receptor-dependent phosphorylation of ERK1/2 was observed in the PFC and cortical neurons. Blockage of dopamine D1, but not D2, receptors in the PFC impaired the long-term recognition memory without affecting short-term memory. Furthermore, c-Fos protein expression was increased in the PFC after exposure to novel objects. Inhibition of protein synthesis in the PFC impaired long-term, but not short-term, recognition memory. These results suggest that the activation of ERK1/2 following stimulation of dopamine D1 receptors in the PFC is necessary for the protein synthesis-dependent long-term retention of recognition memory.
Materials and Methods
Animals
Male ICR mice (7 wk old) were obtained from Japan SLC Inc. All animal care and use were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Kanazawa University with an effort to minimize the number of animals used and their suffering.
Drugs
The following drugs were used in this study: anisomycin, PD98059, SKF38393, and S-(−)-raclopride L(+)-tatrate from Sigma and SCH23390 hydrochloride from TOCRIS. SL327 was kindly provided by DuPont Pharmaceutical Company. SKF38393, SCH23390, and raclopride were dissolved in saline. Anisomycin was dissolved in a minimal volume of 3 M HCl and the solution was adjusted to pH 7.2 and brought to a concentration of 100 mg/mL by addition of 3 M NaOH (Quevedo et al. 2004). PD98058 and SL327 were dissolved in dimethylsulfoxide (Einat et al. 2003; Kamei et al. 2006).
SKF38393 was administered 30 min before decapitation in a volume of 0.1 mL/10 g body weight. SL327 was administered 60 min before the training session for the novel object recognition test in a volume of 0.02 mL/10 g body weight. For microinjection into the PFC or hippocampus, a 28-gauge injection cannula (Eicom) cut to extend 1.0 mm beyond a guide cannula was inserted through a guide cannula. PD98059 (2 μg/1.0 μL/side), SCH23390 (1 μg/0.5 μL/side), raclopride (5 μg/0.5 μL/side), or anisomycin (50 μg/0.5 μL/side) was injected bilaterally over a 5-min period, 20, 15, 15, and 30 min, respectively, before the training session in the novel object recognition test.
Surgery
Under anesthesia (pentobarbital 50 mg/kg, i.p.), mice were placed in a stereotaxic apparatus and bilaterally implanted with the guide cannula (11.6 mm, 0.4 mm in inner diameter, 0.5 mm in outer diameter; Eicom) in the PFC (coordinates: +1.5 mm anteroposterior, ±0.5 mm mediolateral from the bregma, −1.2 mm dorsoventral from the skull) or hippocampus (coordinates: −2.2 mm anteroposterior, ±2.0 mm mediolateral from the bregma, −1.2 mm dorsoventral from the skull) according to the atlas of Franklin and Paxinos (1997). A dummy cannula (0.3 mm in diameter; Eicom) was left in place throughout the experiment. Five days after the operation, mice were subjected to the novel object recognition test.
Novel object recognition test
The novel object recognition test was done according to previously reported methods (Tang et al. 1999; Nagai et al. 2003). The experimental apparatus consisted of a Plexiglas open-field box (30 × 30 × 35 cm high), the floor of which was covered in sawdust. The apparatus was located in a sound-attenuated room and was illuminated with a 20-W bulb.
The procedure for the novel object recognition test consisted of three different sessions: habituation, training, and retention. Each mouse was individually habituated to the box, with 10 min of exploration in the absence of objects each day for three consecutive days (habituation session, day 1–3). During the training session, two different novel objects were symmetrically fixed to the floor of the box, 8 cm from the walls, and each animal was allowed to explore in the box for 10 min (day 4). The objects were constructed from a golf ball, wooden column, and wall socket, which were different in shape and color but similar in size. An animal was considered to be exploring the object when its head was facing the object or it was touching or sniffing the object. The time spent exploring each object was recorded. After training, mice were immediately returned to their home cages. During the retention sessions, the animals were placed back into the same box 1 or 24 h (day 5) after the training session, but with one of the familiar objects used during training replaced by a novel object. The animals were then allowed to explore freely for 5 min and the time spent exploring each object was recorded. Throughout the experiments, the objects were used in a counter-balanced manner in terms of their physical complexity and emotional neutrality. A preference index, a ratio of the amount of time spent exploring any one of the two objects (training session) or the novel object (retention session) over the total time spent exploring both objects, was used to measure cognitive function.
Preparation of neuronal cultures
Primary neuronal cultures were prepared from cerebral cortex of 15-d-old embryonic mice as originally described by di Porzio et al. (1980) with several modifications. Cerebral cortex was dissected from embryonic ICR mice and incubated with Versene (GIBCO BRL) at room temperature for 12 min. Cells were then mechanically dissociated with a fire-narrowed Pasteur pipette in the culture medium, and plated at a density of 1.5 × 105 cells/cm2 on a 6-well or 24-well dish (IWAKI) in basal Dulbecco’s Modified Eagle’s Medium (DMEM)/Nutrient Mixture F-12 (DMEM/F-12, GIBCO BRL) supplemented with 10% fetal calf serum (FCS, Dainippon Pharmaceutical Co.), 33 mM glucose, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL ostreptomycin, 5 mM HEPES, and 0.11% sodium bicarbonate. Prior to use, dishes were sequentially coated with 20 μg/mL of poly-L-lysine. After 8 h in culture, the culture medium was replaced with basal DMEM/F-12 containing 5% FCS, 33 mM glucose, 2 mM glutamine, 100 of U/mL penicillin, 100 μg/mL of streptomycin, 5 mM HEPES, 0.11% sodium bicarbonate, 25 of μg/mL transferrin, 250 ng/mL of insulin, 0.5 pM β-estradiol, 1.5 nM triiodothyronine, 10 nM progesterone, 4 ng/mL of sodium seleniate, and 50 μM putrescine. Cells were treated with 10 μM cytosine arabinoside (Ara-C) for 24 h during 2 to 3 d in vitro (DIV). Cultures were kept in serum-free medium, basal DMEM supplemented with 25.5 mM glucose, 0.5 mM glutamine, 100 U/mL openicillin, 100 μg/mL streptomycin, 0.11% sodium bicarbonate, 50 μg/mL transferrin, 500 ng/mL insulin, 1 pM β-estradiol, 3 nM triiodothyronine, 20 nM progesterone, 8 ng/mL sodium seleniate, and 100 μM putrescine after 3 DIV. The culture medium was replaced with freshly prepared culture medium of the same composition every 3 d. Cultures were always maintained at 37°C in a 5% CO2/95% air-humidified incubator.
Cortical neurons cultured for 8–10 DIV were washed with recording medium containing 129 mM NaCl, 4 mM KCl, 2 mM CaCl2, 4.2 mM glucose, and 10 mM HEPES (pH 7.4) twice, then stimulated with SKF38393 for 30 min. SCH23390 or SL327 was added to the culture 15 min prior to SKF38393. The stimulation was stopped by washing with ice-cold phosphate-buffered saline (PBS) and Western blotting analysis was conducted.
Western blotting
We examined whether the activation of ERK1/2 occurred in the brain of mice that were exposed to the novel objects during the training. For this experiment, mice were habituated to the test box in the absence of objects for 3 d (day 1–3), and sacrificed immediately after a 10-min exposure to the two novel objects in the training session on day 4. Control mice were placed in the open field in the absence of objects on day 4. Brains were removed rapidly, and the PFC and hippocampus were dissected out on an ice-cold plate. Each tissue sample was frozen quickly and stored in a freezer at −80°C until assayed.
The phosphorylation of ERK1/2 was examined by Western blotting as described previously (Mizoguchi et al. 2004). Samples were homogenized at 4°C in a lysis buffer composed of 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mM sodium orthovanadate, 0.1% SDS, 1% sodium deoxycholate, 0.5 mM dithiothreitol, 10 mM sodium pyrophosphate decahydrate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL of aprotinin, 10 μg/mL of leupeptin, and 10 μg/mL of pepstatin. The protein concentration was determined using a DC Protein Assay Kit (Bio-Rad Laboratories), and 20 μg of protein was boiled in a sample buffer (0.125 M Tris-HCl at pH. 6.8, 2% SDS, 5% glycerol, 0.002% bromophenol blue, and 5% 2-mercaptoethanol), applied onto a 10% SDS-polyacrylamide gel, and subsequently transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with 3% BSA in Tris-buffered saline-Tween 20 (TBS-T: 10 mM Tris-HCl at pH. 7.5, 100 mM NaCl, and 1% Tween-20), and incubated with anti-phospho-ERK1/2 (phospho-p44/42 MAPK [Thr202/Tyr204] [E10] Monoclonal Antibody) (1:1000, Cell Signaling Technology) at 4°C overnight. After incubation with a horseradish peroxidase-conjugated anti-mouse IgG (1:2000, KPL) for 1 h, the immune complex was detected using ECL plus Western blotting detection reagents (Amersham Biosciences). The intensities of the bands on the membranes were analyzed by densitometry (ATTO). To calculate the amount of phosphorylated versus total protein, the same membranes were stripped with a stripping buffer (2% SDS, 100 mM 2-mercaptoethanol, and 62.5 mM Tris-HCl at pH 6.8) at 50°C for 30 min, incubated with anti-ERK1/2 (1:5000, anti-MAP Kinase 1/2, Upstate Biotechnology), and treated as described above.
Because there was no change in the levels of total ERK1/2, values of phosphorylated ERK1/2 were normalized to the values of total ERK1/2. All the data from Western blotting are expressed as a percentage of the control.
c-Fos immunohistochemistry
c-Fos immunostaining was performed as described previously (Takahashi et al. 2006). For this experiment, mice were habituated to the test box in the absence of objects for 3 d (day 1–3), and exposed to the two novel objects for 10 min in the training session on day 4. Control mice were placed in the open field in the absence of objects on day 4. Because Fos expression was shown to occur from 1 to 4 h after a single short stimulation (Herdegen and Leah 1998), animals were deeply anesthetized with sodium pentobarbital (50 mg/kg) 2 h after the behavioral test as described above, and transcardially perfused with ice-cold 0.1 M phosphate buffer, followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brains were removed, post-fixed in the same fixative for 2 h, and then cryoprotected in 30% sucrose in 0.1 M phosphate buffer. Frozen serial coronal sections (20 μm) of the entire brain were made and incubated with 10% normal goat serum and 0.1% Triton X-100 in 0.1 M phosphate buffer, and then incubated with rabbit anti-c-Fos antibody (1:200, Santa Cruz Biotechnology) for 24 h at 4°C. They were washed with phosphate buffer and incubated with biotinylated goat anti-rabbit antibody (1:200, Vector Laboratories) at room temperature for 1 h. The sections were washed and processed with avidin-biotinylated horseradish peroxidase complex (Vector ABC kit, Vector Laboratories), and the reaction was visualized using diaminobenzidine.
Quantitative analysis of c-Fos immunohistochemistry
To quantify the number of c-Fos-positive cells in the brain, we examined the sections with a light microscope (Zeiss, HB050) and photographed them with Axio Vision (Zeiss). Three sequential sections for PFC, located according to the atlas of Franklin and Paxinos (1997), were examined for counting the c-Fos-positive cells. In each section, we defined a region of interest (ROI), the size of which was 360 × 360 μm, using software (Win Roof, Mitani Corp.). The counting of c-Fos-positive cells was performed by an individual blind to the treatment conditions. The average of the three determinations was used for statistical analysis.
Statistical analysis
All data were expressed as the mean ± SE. Statistical significance was determined using a one-way analysis of variance (ANOVA), followed by the Bonferroni test when F ratios were significant (P < 0.05). The Mann-Whitney U-test was used for two-group comparisons.
Acknowledgments
We thank DuPont Pharmaceutical Company for providing SL327. This study was supported in part by Grants-in Aid for Scientific Research (No. 17790056) and for the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, a Grant-in-Aid for Health Science Research from the Ministry of Health, Labor, and Welfare of Japan, and grants from the Smoking Research Foundation, Japan, Mitani Foundation.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.461407
References
- Abel T., Lattal K.M. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P., Brown M.W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Akirav I., Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb. Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Atkins C.M., Selcher J.C., Petraitis J.J., Trzaskos J.M., Sweatt J.D. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Barker G.R., Warburton E.C., Koder T., Dolman N.P., More J.C., Aggleton J.P., Bashir Z.I., Auberson Y.P., Jane D.E., Brown M.W. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: Implications for underlying plasticity mechanisms. J. Neurosci. 2006;26:3561–3566. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva M.M., Coscia C.J. Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals. 2002;11:34–44. doi: 10.1159/000057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D.E., Hazvi S., Rosenblum K., Seger R., Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J. Neurosci. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J., Jensen H.C., Bevins R.A. Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behav. Brain Res. 1999;103:35–44. doi: 10.1016/s0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Blum S., Moore A.N., Adams F., Dash P.K. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial learning. J. Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R., Gnesutta N., Minichiello L., White G., Roylance A.J., Herron C.E., Ramsey M., Wolfer D.P., Cestari V., Rossi-Arnaud C., et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- Brown M.W., Aggleton J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Chandler L.J., Sutton G., Dorairaj N.R., Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J. Biol. Chem. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen J., Rusnak M., Luedtke R.R., Sidhu A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J. Biol. Chem. 2004;279:39317–39330. doi: 10.1074/jbc.M403891200. [DOI] [PubMed] [Google Scholar]
- Cobb M.H., Goldsmith E.J. How MAP kinases are regulated. J. Biol. Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Dash P.K., Hebert A.E., Runyan J.D. A unified theory for systems and cellular memory consolidation. Brain Res. Brain Res. Rev. 2004;45:30–37. doi: 10.1016/j.brainresrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Davis H.P., Squire L.R. Protein synthesis and memory: A review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- di Porzio U., Daguet M.D., Glowinski J., Prochiantz A. Effect of striatal cells on in vitro maturation of mesencephalic dopaminergic neurones grown in serum-free conditions. Nature. 1980;288:370–373. doi: 10.1038/288370a0. [DOI] [PubMed] [Google Scholar]
- Egan M.F., Weinberger D.R. Neurobiology of schizophrenia. Curr. Opin. Neurobiol. 1997;7:701–707. doi: 10.1016/s0959-4388(97)80092-x. [DOI] [PubMed] [Google Scholar]
- Einat H., Yuan P., Gould T.D., Li J., Du J., Zhang L., Manji H.K., Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M., Fletcher P.J., Drago J., Sibley D.R., O'Dowd B.F., George S.R. Spatial learning deficit in dopamine D1 receptor knockout mice. Eur. J. Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Farde L., Halldin C., Stone-Elander S., Sedvall G. PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology. 1987;92:278–284. doi: 10.1007/BF00210831. [DOI] [PubMed] [Google Scholar]
- Franklin J.B.J., Paxinos G.T. The mouse brain: In stereotaxic coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Gaspar P., Bloch B., Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: Cellular localization in different classes of efferent neurons. Eur. J. Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gilbert P.E., Kesner R.P. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learn. Mem. 2003;10:525–530. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Lidow M.S., Smiley J.F., Williams M.S. The anatomy of dopamine in monkey and human prefrontal cortex. J. Neural Transm. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- Greba Q., Gifkins A., Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Hebert A.E., Dash P.K. Extracellular signal-regulated kinase activity in the entorhinal cortex is necessary for long-term spatial memory. Learn. Mem. 2002;9:156–166. doi: 10.1101/lm.48502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T., Leah J.D. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hotte M., Naudon L., Jay T.M. Modulation of recognition and temporal order memory retrieval by dopamine D1 receptor in rats. Neurobiol. Learn. Mem. 2005;84:85–92. doi: 10.1016/j.nlm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Ernst W.H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei H., Nagai T., Nakano H., Togan Y., Takayanagi M., Takahashi K., Kobayashi K., Yoshida S., Maeda K., Takuma K., et al. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol. Psychiatry. 2006;59:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kelleher R.J., III, Govindarajan A., Jung H.Y., Kang H., Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelly A., Laroche S., Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Sesack S.R., Levey A.I., Rosenberg D.R. Dopamine axons in primate prefrontal cortex: Specificity of distribution, synaptic targets, and development. Adv. Pharmacol. 1998;42:703–706. doi: 10.1016/s1054-3589(08)60845-5. [DOI] [PubMed] [Google Scholar]
- Lidow M.S., Goldman-Rakic P.S., Gallager D.W., Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Mayr B.M., Canettieri G., Montminy M.R. Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc. Natl. Acad. Sci. 2001;98:10936–10941. doi: 10.1073/pnas.191152098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister A.K., Katz L.C., Lo D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McClelland J.L., McNaughton B.L., O'Reilly R.C. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Memory—A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller C.A., Marshall J.F. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., Yamada K., Mizuno M., Mizuno T., Nitta A., Noda Y., Nabeshima T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharmacol. 2004;65:1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- Mumby D.G. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behav. Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Murray E.A., Richmond B.J. Role of perirhinal cortex in object perception, memory, and associations. Curr. Opin. Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nagai T., Yamada K., Kim H.C., Kim Y.S., Noda Y., Imura A., Nabeshima Y., Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: A role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Nagai T., Kamei H., Dohniwa M., Takayanagi M., Suzuki M., Matsuya T., Nabeshima T., Takuma K., Yamada K. Involvement of hippocampal extracellular signal-regulated kinase 1/2 in spatial working memory in rats. Neuroreport. 2006;17:1453–1457. doi: 10.1097/01.wnr.0000233095.74913.88. [DOI] [PubMed] [Google Scholar]
- Pearson G., Robinson F., Beers Gibson T., Xu B.E., Karandikar M., Berman K., Cobb M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Phillips A.G., Ahn S., Floresco S.B. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J. Neurosci. 2004;24:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post G.R., Brown J.H. G protein-coupled receptors and signaling pathways regulating growth responses. FASEB J. 1996;10:741–749. doi: 10.1096/fasebj.10.7.8635691. [DOI] [PubMed] [Google Scholar]
- Quevedo J., Vianna M.R., Martins M.R., Barichello T., Medina J.H., Roesler R., Izquierdo I. Protein synthesis, PKA, and MAP kinase are differentially involved in short- and long-term memory in rats. Behav. Brain Res. 2004;154:339–343. doi: 10.1016/j.bbr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Ragozzino M.E., Detrick S., Kesner R.P. The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol. Learn. Mem. 2002;77:29–43. doi: 10.1006/nlme.2001.4003. [DOI] [PubMed] [Google Scholar]
- Runyan J.D., Dash P.K. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol. Learn. Mem. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Runyan J.D., Moore A.N., Dash P.K. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., Atkins C.M., Swank M.W., Bauer E.P., Sweatt J.D., LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Floresco S.B., Phillips A.G. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher J.C., Atkins C.M., Trzaskos J.M., Paylor R., Sweatt J.D. A necessity for MAP kinase activation in mammalian spatial learning. Learn. Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nagai T., Kamei H., Maeda K., Matsuya T., Arai S., Mizoguchi H., Yoneda Y., Nabeshima T., Takuma K., et al. Neural circuits containing pallidotegmental GABAergic neurons are involved in the prepulse inhibition of the startle reflex in mice. Biol. Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.06.035. (in press) [DOI] [PubMed] [Google Scholar]
- Tang Y.P., Shimizu E., Dube G.R., Rampon C., Kerchner G.A., Zhuo M., Liu G., Tsien J.Z. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Tyler W.J., Alonso M., Bramham C.R., Pozzo-Miller L.D. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E., Corvol J.C., Pages C., Besson M.J., Maldonado R., Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E., Caboche J., Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: A molecular substrate for learning and memory? Mol. Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- Valjent E., Pages C., Herve D., Girault J.A., Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Vossler M.R., Yao H., York R.D., Pan M.G., Rim C.S., Stork P.J.S. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Walz R., Roesler R., Quevedo J., Rockenbach I.C., Amaral O.B., Vianna M.R., Lenz G., Medina J.H., Izquierdo I. Dose-dependent impairment of inhibitory avoidance retention in rats by immediate post-training infusion of a mitogen-activated protein kinase kinase inhibitor into cortical structures. Behav. Brain Res. 1999;105:219–223. doi: 10.1016/s0166-4328(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Walz R., Roesler R., Quevedo J., Sant'Anna M.K., Madruga M., Rodrigues C., Gottfried C., Medina J.H., Izquierdo I. Time-dependent impairment of inhibitory avoidance retention in rats by posttraining infusion of a mitogen-activated protein kinase kinase inhibitor into cortical and limbic structures. Neurobiol. Learn. Mem. 2000;73:11–20. doi: 10.1006/nlme.1999.3913. [DOI] [PubMed] [Google Scholar]
- West A.E., Chen W.G., Dalva M.B., Dolmetsch R.E., Kornhauser J.M., Shaywitz A.J., Takasu M.A., Tao X., Greenberg M.E. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B.D., Bussey T.J. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 2005a;25:4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B.D., Bussey T.J. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 2005b;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.R., Seamans J.K., Gorelova N. Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology. 1999;21:161–194. doi: 10.1016/S0893-133X(98)00112-2. [DOI] [PubMed] [Google Scholar]
- Zanassi P., Paolillo M., Feliciello A., Avvedimento E.V., Gallo V., Schinelli S. cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. J. Biol. Chem. 2001;276:11487–11495. doi: 10.1074/jbc.M007631200. [DOI] [PubMed] [Google Scholar]