Abstract
Little is known about the molecular anomalies involved in the development and progression of malignancy of pancreatic endocrine tumors (PETs). A recently identified member of the Ras family, Ras homologue member I (ARHI), has been shown to be involved in breast, ovary, and thyroid carcinogenesis. Unlike other members, it acts as a tumor suppressor gene that inhibits cell growth. Here we analyzed the mRNA expression of ARHI in 52 primary PETs and 16 normal pancreata using quantitative reverse transcription-polymerase chain reaction. ARHI expression showed a statistically significant difference between either normal pancreas or well-differentiated endocrine tumors (WDET) and poorly differentiated endocrine carcinomas (PDECs) (P < .001 and P < .001, respectively). Moreover, ARHI expression among WDEC samples was more heterogeneous than in WDET, with several tumors showing level of expression analogous to that observed in PDECs. A significant correlation between lower ARHI expression and shorter survival (P = .020) was identified, and a low ARHI expression was associated to a shorter time to progression (P < .001), even considering the proliferation index Ki67 in the multivariate analysis. ARHI is involved in PET progression. Its mRNA expression seemed to be a prognostic factor for disease outcome and, in association with the proliferative index Ki67, a predictor for a rapid tumor relapse.
Keywords: ARHI, pancreatic endocrine tumor, tumor differentiation, survival, time to progression
Introduction
Pancreatic endocrine tumors (PETs) are a heterogeneous group of neoplasms that are clinically classified as functioning or nonfunctioning according to the presence of symptoms due to hormone hypersecretion. According to the World Health Organization classification [1], PETs, whether functioning or not, are classified into three categories: 1) well-differentiated endocrine tumors (WDETs), 2) well-differentiated endocrine carcinomas (WDECs), and 3) poorly differentiated endocrine carcinomas (PDECs). The WDET category is further distinguished into two subgroups with either benign or uncertain clinical behavior [1]. Carcinomas are characterized by the presence of invasion or metastases, and among them PDECs are highly aggressive and show a poorer outcome compared with WDECs [1]. To date, little is known about the molecular pathways and anomalies involved in PET development and progression [2].
Ras homologue member I (ARHI) is one of the first identified tumor suppressors belonging to the Ras superfamily. It encodes a 26-kDa GTPase with 50% to 60% amino acid homology to Ras but exerts opposite functions that inhibit cell growth, motility, and invasion [3–5]. Notably, it has been shown that ARHI underexpression correlated with breast tumor progression as well as reduced progression-free survival in ovarian cancer [6,7].
In the present work, we analyzed the expression of ARHI in 52 PETs and 16 normal pancreata to assess possible clinicalpathological correlations with tumor differentiation, proliferation rate, and clinical behavior including time to progression (TTP) and survival.
Materials and Methods
Materials
The study involved 52 primary PETs (22WDETs, 26WDECs, and 4 PDECs), obtained from patients that underwent either explorative or radical surgery with their informed consent (Table W1), and 16 normal samples (Table W2). These included 12 pancreatic bulk tissues and four human pancreatic islets of Langerhans cell preparations [8]. PETs were diagnosed by histopathological and cell marker analysis and classified according to World Health Organization criteria [1].
Total RNA was prepared from 10 to 20 cryostat sections (40 µm thick) of snap-frozen tissue, checking the cellularity every four sections. Tissue sections were placed in 4 M guanidine thiocyanate containing 0.1 M 2-mercaptoethanol and centrifuged through a CsCl2 gradient. RNA quality was assessed by using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). cDNA was synthesized from 1 µg of total RNA using random primers and the Superscript II reverse transcription kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Quantitative Reverse Transcription Polymerase Chain Reaction
Quantitative Reverse Transcription polymerase chain reaction (PCR) mRNA expression analysis was performed on ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) using SYBR green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. Oligonucleotide primers used were the following: ARHI-F, AGAAAGGGGTCTCCTGCTG, and ARHI-R, GCAGCTTCTGTTCCTTGGAG; β-actin-F, GGAGTCCTGTGGCATCCACG, and β-actin-R, CTAGAAGCATTTGCGGTGGA. β-Actin transcript level (RefSeq ID NM_001101) was used to normalize the samples.
Calibration curves of each couple of primers were obtained by serial dilution of cDNA. Expression data were analyzed by the comparative threshold cycle (Ct) method accordingly to User Bulletin No. 2 (Applied Biosystems). Results were expressed in terms of the ΔΔCt value and obtained as follows: ΔCtARHI = CtARHI-Ctβ-actin and ΔΔCtARHI = ‖‖ΔCtARHI-max(ΔCtARHI)‖‖, where CtARHI and Ctβ-actin represent the comparative threshold cycles for ARHI and β-actin, respectively. All experiments were performed in duplicate.
Time to Progression and Survival Evaluation
Patients were monitored every 3- months with contrast-enhanced CTscan, clinical evaluation of symptoms and body weight, and measurement of blood parameters and tumor markers. Time to progression was defined as the interval from the surgery until disease progression, as previously described [9]. Survival was calculated from the date of diagnosis to the date of death, and only deaths from the disease were considered. ARHI expression was categorized by applying a cutoff level defined as the lowest ΔΔCt measured in the normal control samples. Therefore, tumors with ΔΔCt below this cutoff were considered to have a low ARHI expression, whereas those with ΔΔCt above the cutoff were considered as having a normal level of expression.
Statistical Analysis
The statistical significance of the differences between subgroups was investigated by either logistic regression analysis or the Mann-Whitney test. Survival and TTP data were analyzed using Kaplan Meier function and the log-rank test for univariate analyses or Cox proportional hazard regression. All P values were two sided and considered significant when less than .05. All the analyses were performed using R software v. 2.1.1 and Survival package (http://www.R-project.org).
Results and Discussion
The Ras homologue member I (ARHI) is a tumor suppressor gene and a member of the Ras family that is able to negatively regulate the Ras/mitogen-activated protein kinases (MAPK) signaling pathway inhibiting cancer cell growth [3]. Wang et al. have shown that ARHI was frequently down-regulated in breast carcinoma and was negatively associated with tumor progression [7]. More interestingly, ARHI expression was associated with expression of p21WAF/CIP1 and prolonged disease-free survival in epithelial ovarian cancer [6]. In addition, it has been shown that ARHI silencing could contribute to the carcinogenesis of follicular thyroid carcinomas, which are the most common endocrine cancers [10].
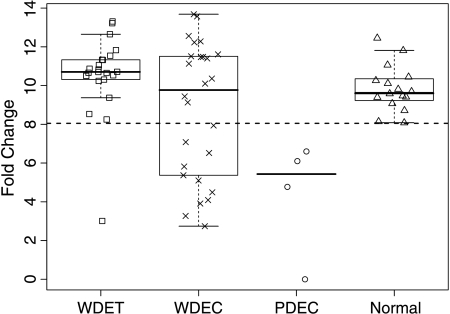
We analyzed the mRNA level of ARHI by quantitative realtime PCR in 52 PETs to identify possible correlations with clinical-pathological parameters (Tables W1 and W3). Logistic regression analysis identified a significant correlation between ARHI expression and tumor differentiation (odds ratio, 0.916; 95% confidence interval, 0.894–0.938; P < .001). In fact, PDEC samples showed a significant lower expression of ARHI compared with normal pancreas and WDET (Mann-Whitney test, P < .001 and P < .001, respectively), whereas no significant difference was observed between WDET and WDEC. However, the expression of ARHI among the WDEC samples was heterogeneous, with several tumors showing levels of expression analogous to those observed in PDEC samples (Figure 1). Notably, patients with WDEC with low ARHI expression showed a median TTP of 30 months versus 49 months observed in WDEC patients with normal ARHI expression, although this difference was not statistically significant.
Figure 1.
Box and whiskers plot of ARHI mRNA expression in 52 PETs and 16 normal pancreatic samples. Dotted line indicate the cutoff level used to distinguish samples with low (below the line) and normal (above the line) ARHI mRNA expression. WDET, well-differentiated tumor; WDEC, welldifferentiated carcinoma; PDEC, poorly differentiated carcinoma.
The correlation between ARHI expression and survival or TTP was tested in 47 and 49 patients, respectively, for which these data were available. A shorter survival was associated with tumor differentiation (P < .001), low ARHI expression (P = .020; Figure W1) and high proliferative index (Ki67 5%; P < .001). The association between survival and Ki67 remained significant when PDECs were excluded from the analysis (P = .016), confirming the reported prognostic value of proliferative index [1,11]. Similarly, a shorter TTP was associated with tumor differentiation (P < .001), reduced ARHI expression (P < .001; Figure W2), and high proliferative index (P < .001). In addition, shorter TTP was found to be associated with low ARHI expression (P = .036) and high proliferation index (P = .001) when only WDET and WDEC were included in the analysis.
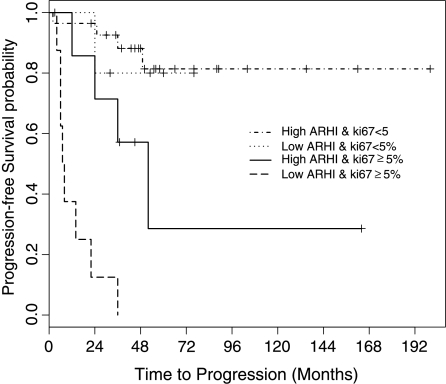
Notably, multivariate analysis showed that ARHI expression was an independent prognostic factor for TTP in association with the proliferative index Ki67 (Cox proportional hazard; Table 1, Figure 2), which is the most informative molecular marker of PEToutcome [11]. In particular, patients with low ARHI expression showed a significantly higher hazard ratio for disease progression compared with those with normal ARHI expression. Moreover, all the seven patients with low ARHI expression and high proliferation index showed tumor progression after a shorter time.
Table 1.
Multivariate Analysis of TTP in PETs.
| Hazard Ratio (95% Confidence Interval) | P | |
| Low ARHI expression | 4.0 (1.5 – 11.1) | .007 |
| Ki67 ≥5% | 8.3 (2.8 – 24.8) | .0002 |
Hazard ratio represents an estimate of the ratio of mortality rate in patients with low ARHI expression versus patients with normal ARHI expression, or patients with proliferation index Ki67 <5% versus patients with Ki67 ≥5%. Cox proportional hazard model was used with simultaneous inclusion of all factors shown.
Figure 2.
Progression free-survival curve; 49 patients with high or low ARHI mRNA expression and Ki-67 proliferation index less than or greater than 5% were considered.
These findings suggest that ARHI is involved in PET progression. In addition, ARHI mRNA level seems to be a prognostic factor for disease outcome and, in association with the proliferative index Ki67, a predictor of a rapid tumor relapse.
Supplementary Material
Abbreviations
- PET
pancreatic endocrine tumor
- WDET
well-differentiated endocrine tumor
- WDEC
well-differentiated endocrine carcinoma
- PDEC
poorly differentiated endocrine carcinoma
- TTP
time to progression
Footnotes
This study was supported by Associazione Italiana Ricerca Cancro, Fondazione Giorgio Zanotto (Verona, Italy), Ministero Università e Ricerca e Ministero Salute (Rome, Italy), Fondazione Cassa di Risparmio di Verona (AS), and European Community FP VI Program grant PL018771.
This article refers to supplementary materials, which are designated by “W” (i.e., Tables W1–W3; Figures W1 and W2) and are available online at www.bcdecker.com.
References
- 1.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 2.Perren A, Komminoth P, Heitz PU. Molecular genetics of gastroenteropancreatic endocrine tumors. Ann N Y Acad Sci. 2004;1014:199–208. doi: 10.1196/annals.1294.021. [DOI] [PubMed] [Google Scholar]
- 3.Luo RZ, Fang X, Marquez R, Liu SY, Mills GB, Liao WS, Yu Y, Bast RC. ARHI is a Ras-related small G-protein with a novel N-terminal extension that inhibits growth of ovarian and breast cancers. Oncogene. 2003;22:2897–2909. doi: 10.1038/sj.onc.1206380. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Luo R, Lu Z, Wei Feng W, Badgwell D, Issa JP, Rosen DG, Liu J, Bast RC., Jr Biochemistry and biology of ARHI (DIRAS3), an imprinted tumor suppressor gene whose expression is lost in ovarian and breast cancers. Methods Enzymol. 2005;407:455–468. doi: 10.1016/S0076-6879(05)07037-0. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Xu F, Peng H, Fang X, Zhao S, Li Y, Cuevas B, Kuo WL, Gray JW, Siciliano M, et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci USA. 1999;96:214–219. doi: 10.1073/pnas.96.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen DG, Wang L, Jain AN, Lu KH, Luo RZ, Yu Y, Liu J, Bast RC., Jr Expression of the tumor suppressor gene ARHI in epithelial ovarian cancer is associated with increased expression of p21WAF1/CIP1 and prolonged progression-free survival. Clin Cancer Res. 2004;10:6559–6566. doi: 10.1158/1078-0432.CCR-04-0698. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Hoque A, Luo RZ, Yuan J, Lu Z, Nishimoto A, Liu J, Sahin AA, Lippman SM, Bast RC, Jr, et al. Loss of the expression of the tumor suppressor gene ARHI is associated with progression of breast cancer. Clin Cancer Res. 2003;9:3660–3666. [PubMed] [Google Scholar]
- 8.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Butturini G, Bettini R, Missiaglia E, Mantovani W, Dalai I, Capelli P, Ferdeghini M, Pederzoli P, Scarpa A, Falconi M. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13:1213–1221. doi: 10.1677/erc.1.01200. [DOI] [PubMed] [Google Scholar]
- 10.Weber F, Aldred MA, Morrison CD, Plass C, Frilling A, Broelsch CE, Waite KA, Eng C. Silencing of the maternally imprinted tumor suppressor ARHI contributes to follicular thyroid carcinogenesis. J Clin Endocrinol Metab. 2005;90:1149–1155. doi: 10.1210/jc.2004-1447. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, Iacono C, Serio G, Zamboni G. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol. 1996;27:1124–1134. doi: 10.1016/s0046-8177(96)90303-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.