Abstract
Angiogenesis plays a critical role in tumor progression in various cancers, including neuroblastoma. We have previously shown that gastrin-releasing peptide (GRP) stimulates neuroblastoma growth and that its cell surface receptors, gastrin-releasing peptide receptors (GRP-R), are overexpressed in advanced-stage human neuroblastomas; however, the effects of GRP on angiogenesis are not clearly elucidated. Interleukin (IL) 8, a proinflammatory chemokine, plays an important role during tumor angiogenesis. Ets transcription factors, such as oncoproteins, cause tumor development and are also known to induce IL-8 expression. In the present study, we found an increased expression of Ets1 in more undifferentiated human neuroblastomas. Stable transfection of SK-N-SH human neuroblastoma cells with Ets1 plasmid resulted in increased IL-8 luciferase activity and IL-8 secretion into cell culture media. Conversely, silencing of Ets1 resulted in a significant decrease in IL-8 secretion in SK-N-SH cells. Moreover, exogenous GRP treatment increased Ets1 (T38) phosphorylation and Ets1 nuclear accumulation, and enhanced Ets1 binding to its DNA consensus sequence, resulting in the stimulation of IL-8 mRNA expression and protein secretion. Our findings demonstrate that GRP upregulates proangiogenic IL-8 expression in an Ets1-dependent manner, suggesting a critical role of this process during GRP-induced neuroblastoma angiogenesis and metastasis.
Keywords: Neuroblastoma, GRP, Ets1, IL-8, angiogenesis
Introduction
Neuroblastoma is the most common extracranial solid tumor in pediatric patients, and its clinical behavior is quite variable, depending on its histopathological features. Angiogenic factors are differentially expressed in neuroblastomas in a pattern suggesting the promotion of a proangiogenic phenotype in more undifferentiated aggressive tumors [1]. Recent studies implicate angiogenesis in the regulation of neuroblastoma growth [2]. The vascularity of neuroblastomas from patients with widely metastatic diseases is significantly higher than that of tumors from patients with local or regional diseases [2–4]. Hence, inhibition of angiogenesis could potentially be a promising approach in the treatment of advanced-stage neuroblastomas.
Neuroblastomas are known to produce and secrete various peptides [5], including gastrin-releasing peptide (GRP) [6]. GRP, the mammalian equivalent of bombesin, is a neuroendocrine peptide that exerts many cellular functions, including a growth-stimulatory effect on various cancer cell types. Recently, we have shown that GRP and its cell surface receptor, gastrin-releasing peptide receptor (GRP-R), are abundantly expressed in human neuroblastomas [6]. Moreover, we have found that clinically more aggressive undifferentiated tumors express an increased level of GRP-R. GRP is produced and secreted by human neuroblastoma cells, therefore acting as an autocrine growth factor to stimulate its cellular growth [6]. However, the effects of GRP on neuroblastoma tumor angiogenesis are unknown.
Tumor growth and metastasis depend on the ability of tumor cells to induce angiogenesis, mediated by the release of angiogenic factors that are secreted by tumor cells. Among them, vascular endothelial growth factor (VEGF) [7] and interleukin (IL) 8 [8] are the most potent angiogenic factors in neuroblastoma. IL-8 is a member of the chemokine family that has pleiotropic function, including roles in angiogenesis [9], chemotaxis [10], and stem cell mobilization [11]. It is expressed by several tumor types, and its expression is regulated primarily at the transcriptional level by transcription factors such as AP-1 and NF-κB [12]. Recently, it has been reported that an IL-8 promoter contains three Ets-binding sites and that myeloid ELF1-like factor, an Ets family transcription factor, specifically binds to the IL-8 promoter in hematopoietic cells [13]. IL-8 is expressed by a number of human malignancies, and its expression correlates with the metastatic potential of tumors. In particular, IL-8 has also been found to be an important angiogenic factor in neuroblastoma [8].
The ets gene family encodes unique transcription regulators that have a common Ets DNA-binding domain. So far, approximately 30 members of the family have been identified in mammals [14]. Ets1 expression levels strongly correlate with the grade of invasiveness and metastasis in preinvasive breast cancer [15,16] and in human colorectal carcinoma [16]. Furthermore, Ets family transcriptional activators and repressors are also involved in angiogenesis. Studies have demonstrated that Ets transcription factors can regulate multiple aspects of the malignant phenotype of many tumor cells, in particular neoangiogenesis and extracellular matrix-regulated cell proliferation, motility, and invasiveness [17].
In this study, we report that oncogenic Ets1 is overexpressed in undifferentiated aggressive human neuroblastomas. GRP, which is expressed and secreted by neuroblastoma cells, stimulates the transcriptional activity of Ets1 by phosphorylation, inducing its translocation into the nucleus and further increasing Ets1 binding to its DNA consensus sequence. This process then results in IL-8mRNA induction and protein secretion, suggesting a role for Ets1 during GRP-induced secretion of proangiogenic IL-8 in neuroblastoma cells.
Materials and Methods
Cell Lines and Reagents
Neuroblastoma cell lines [SK-N-SH, IMR-32, BE(2)-C, SK-N-MC, and SH-SY5Y] were purchased from the American Type Culture Collection (Manassas, VA). LAN-1 was a gift from Dr. Robert C. Seeger (University of Southern California, Los Angeles, CA). GRP peptide was from Bachem (Torrance, CA) and was used at a concentration of 10-7 M in all treatments. All reagents were from Sigma (St. Louis, MO), unless otherwise specified.
Expression Constructs and Small Interfering RNA (siRNA)
IL-8 promoter luciferase plasmid was kindly provided by Dr. Krach Michael (Institute of Pharmacology, Medical School Hannover, Hanover, Germany). Constructs GAL4-Ets1 and 5X GAL-Luc were gifts from Dr. V. Fafeur (Institute de Biologie de Lille, Lille, France). Ets1 expression plasmid (pSG5p51) was a gift from Dr. Runzhao Li (Laboratory of Cancer Genomics, Medical University of South Carolina, Charleston, SC). Ets1 siRNA vector (TranSilent Ets1 siRNA vector) and its siRNA control vector were purchased from Panomics (Redwood City, CA).
Cell Culture, Transfection, and Reporter Luciferase Assays
Cells were maintained in RPMI 1640 medium with l-glutamine (Cellgro Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Sigma). The cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were transfected with plasmid DNA in 24-well plates using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Reporter luciferase activity assays were performed with a Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI) after transfection for indicated times.
Immunohistochemistry
Immunohistochemical staining was performed according to the protocol provided by DAKO EnVision+ System (Dako Corporation, Carpinteria, CA). Formalin-fixed paraffin-embedded sections (4 µm) were dewaxed in xylene and rehydrated through graded alcohol to distilled water. The sections were subjected to antigen retrieval by boiling in a microwave for 20 minutes in a 0.01 M sodium citrate buffer (pH 6.0). Endogenous peroxidase was blocked by treating with blocking buffer for 5 minutes. Sections were blocked with 5% bovine serum albumin in Tris-buffered saline (TBS) buffer for 15 minutes before incubation with antibody. The primary rabbit polyclonal antibody to Ets1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was applied at a dilution of 1:500 and incubated overnight at 4°C. After incubation, the slides were washed thrice with TBS for 5 minutes each. Then the sections were incubated with secondary antibody conjugated with peroxidase for 30 minutes at room temperature (RT). Antibody-antigen complexes were detected by color changes on the addition of diaminobenzidine. Counterstaining was performed with hematoxylin. Negative controls were obtained using normal serum, instead of the primary antibody.
Immunofluorescent Staining
SK-N-SH cells were grown on coverslips in a 12-well plate, fixed in 4% paraformaldehyde, and permeabilized in cold methanol for 5 minutes. Cells were then incubated with primary antibodies against Ets1 (1:500) for 1 hour at RT. Cells were washed and treated with Rhodamin-conjugated goat antirabbit secondary antibody (Molecular Probes, Eugene, OR) for 1 hour at RT and then incubated with 4′,6′-diamidino-2-phenylindole (DAPI) for 5 minutes at RT. Images were obtained using a Nikon Eclipse E600 (Nikon Instruments, Inc., Melville, NY).
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed with EMSA Gel-Shift Kit (Panomics) according to the manufacturer's protocol. Ets/PU1 oligonucleotides (5′-AGTATGTCTAGACTGA-3′) were labeled with biotin, mixed with nuclear proteins in a binding buffer, and incubated with 2.5 µg of nuclear protein for 20 minutes at RT. Protein/DNA complexes were resolved in 6% DNA Retardation Gels (Invitrogen) using 0.5x Trisborated-EDTA buffer.
Western Blot Analysis
Cells were cultured in tissue culture dishes for experiments, unattached cells were removed by rinsing once with phosphate-buffered solution, and attached cells were scraped from culture dishes, pelleted, and resuspended in cell lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 Ég/ml leupeptin, and 1 mM PMSF added before use). Protein concentrations were determined, and aliquots of each sample containing equal amounts of protein were diluted into a sodium dodecyl sulfate sample buffer. Proteins were run on 4% to 12% NuPAGE Novex Bis-Tris Gels (Invitrogen), transferred to polyvinylidene difluoride membranes, and probed with antibodies. Bands were visualized by an enhanced chemiluminescent detection system ECL plus according to the manufacturer's instructions (Amersham, Inc., Piscataway, NJ).
IL-8 Secretion Assay
Human neuroblastoma SK-N-SH cells were seeded at 1 x 105 cells/ml per well in 12-well plates and incubated in RPMI 1640 medium with 10% FBS overnight. Cells were then washed with media without FBS and treated with GRP (10-7 M) for the indicated times. Cell culture supernatants were collected after each experiment and centrifuged to remove cells and debris. IL-8 in the supernatants of cultured cells was measured by enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer's instructions (Pierce Biotechnology, Inc., Rockford, IL).
Statistical Analysis
Each set of experiments was repeated at least thrice. Values are given as mean ± SEM. Conditions were compared using Student's paired t test. One-way analysis of variance on the ranks for repeated measures was performed for multiple comparisons. Differences were considered significant when P < .05.
Results
Increased Ets1 Expression in Poorly Differentiated Human Neuroblastomas
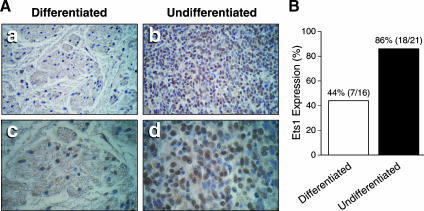
The malignant behavior of neuroblastoma correlates with the degree of tumor cell differentiation, where poorly differentiated tumors are aggressive and often refractory to treatment modalities [1]. A typical poorly differentiated neuroblastoma is characterized by small cells with large round nuclei and scant cytoplasm [18]. In contrast, well-differentiated neuroblastomas (i.e., ganglioneuroma and ganglioneuroblastoma) are heterogeneous, with large mature ganglion cells. In the present study, we analyzed 21 poorly differentiated and 16 well-differentiated human neuroblastomas by immunohistochemistry. Ets1 expression was found in 18 of 21 poorly differentiated neuroblastomas, and in 7 of 16 differentiated specimens (Figure 1A). Interestingly, Ets1 protein was weakly expressed in both the nuclei and the cytoplasm of Ets1-positive differentiated cells (Figure 1A, panels a and c). In contrast, a strong Ets1 expression was found in undifferentiated cell nuclei (brown stain; Figure 1A, panels b and d), indicating an inverse correlation between cellular differentiation and Ets1 expression in human neuroblastomas. Additionally, Ets1 expression was also detected in all six neuroblastoma cell lines examined at various levels (data not shown).
Figure 1.
Ets1 expression in human neuroblastomas. (A) Paraffin-embedded human neuroblastoma sections were stained with an anti-Ets1 antibody visualized by diaminobenzidine staining. Ets1 was strongly expressed in undifferentiated neuroblastoma cells (panels b and d), whereas weakly stained Ets1 was found in well-differentiated neuroblastoma sections (panels a and c). Original magnification: x200 for panels (a) and (b); x400 for panels (c) and (d). (B) Ets1 protein was expressed in 18 of 21 (86%) undifferentiated tumor sections, and in 7 of 16 (44%) differentiated tumor sections.
Ets1 Upregulates IL-8 Transcription and Secretion in Neuroblastoma Cells
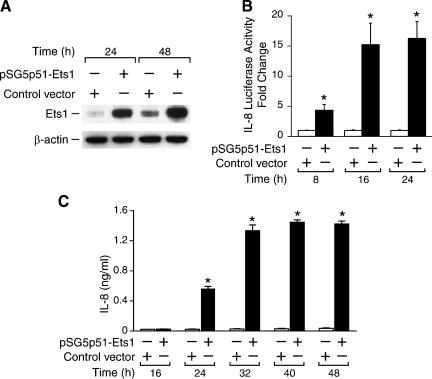
To detect whether Ets1 contributes to angiogenesis in neuroblastomas, SK-N-SH cells were transiently transfected with Ets1 plasmid. The supernatants and cell lysates were collected after transfection at various time points. Ets1 protein expression was significantly increased at 24 and 48 hours after transfection, as confirmed by Western blot analysis (Figure 2A). To determine the critical angiogenic molecules regulated by Ets1, a focused angiogenesis gene array (SuperArray Bioscience, Frederick, MD) was performed. Our array results showed that Ets1 overexpression increased IL-8 mRNA expression (data not shown). To validate this finding, we then performed IL-8 promoter reporter assays by cotransfecting SK-N-SH cells with an IL-8 promoter luciferase vector along with Ets1 plasmid. Ets1 increased IL-8 promoter activity by four-fold at 8 hours and by approximately 15-fold at 16 hours when compared to control cells transfected with an empty vector (Figure 2B), thus confirming the gene array results. Furthermore, we measured IL-8 secretion in culture supernatants by ELISA. Cells were transfected with either Ets1 expression or control plasmid. Our results showed that IL-8 secretion was significantly increased at 24 hours and reached peak stimulation at 40 hours after transfection in cells overexpressing Ets1 (Figure 2C).
Figure 2.
Ets1 regulates IL-8 expression and secretion in SK-N-SH cells. (A) Cells were transfected with the Ets1 expression plasmid, and then Western blot analysis was performed. (B) IL-8 promoter transcriptional activity was enhanced by cotransfection with Ets1 expression plasmid (mean ± SEM; *P < .05 vs control vector). (C) IL-8 secretion into a culture supernatant was increased by Ets1 overexpression (mean ± SEM; *P < .05 vs control vector).
GRP Induces Ets1 Phosphorylation and Subcellular Localization
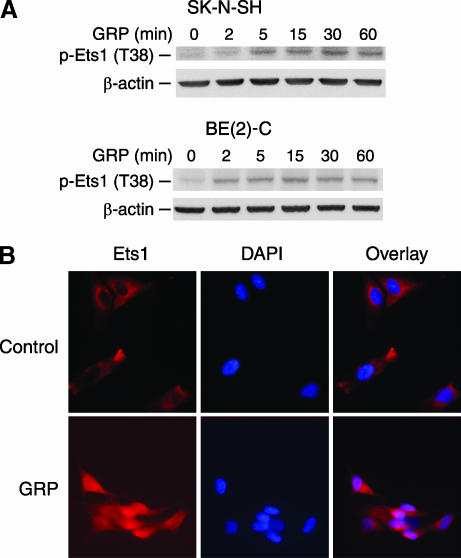
To investigate the effect of GRP on Ets1 activity, neuroblastoma cells were treated with GRP, and Ets1 phosphorylation was analyzed by Western blot analysis using a specific antibody against phosphorylated Ets1 (pT38). Ets1 was rapidly phosphorylated at 5 minutes after GRP treatment, and this persisted up to 60 minutes in SK-N-SH cells (Figure 3A). A similar activation of Ets1 was noted after GRP treatment in another neuroblastoma cell line, BE(2)-C. Phosphorylation of threonine-38 residue strongly increases the transcriptional activity of Ets1 [19]. Furthermore, to determine whether GRP treatment affects Ets1 intracellular localization, we performed immunocytochemical analysis. The cells grown on coverslips were fixed and stained with Ets1-specific antibody; DAPI was used for nuclear staining. Our results showed that GRP-treated cells demonstrate an intense nuclear accumulation of Ets1 within 1 hour when compared to control cells with Ets1 noted in the cytoplasm (Figure 3B).
Figure 3.
Ets1 phosphorylation and nuclear localization after GRP treatment. (A) SK-N-SH cells were serum-starved overnight and then treated with GRP (10-7 M). Induction of Ets1 phosphorylation was detected after GRP treatment in both SK-N-SH and BE(2)-C cells by Western blot analysis. (B) For immunofluorescent staining, SK-N-SH cells were seeded on coverslips and serum-starved overnight. Ets1 protein was localized predominantly in the nuclei at 1 hour (bottom panels) after GRP treatment (10-7 M) when compared to controls (top panels). DAPI specifically stained for the DNA of nuclei.
GRP Increases Ets1 Binding to Its DNA Consensus Sequence and Ets1 Transactivation
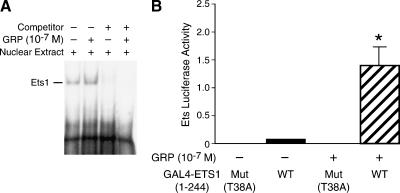
So far, we have shown that GRP induces Ets1 phosphorylation and nuclear localization. We next assessed the effect of GRP on Ets1 transcription factor activity. EMSA was performed to examine the binding of nuclear Ets1 to its DNA consensus sequence in vitro. Sequence-specific binding of Ets1 to its biotin-labeled probe was observed. Ets1 DNA-binding activity was increased with GRP treatment (Figure 4A, lane 2); this was competitively blocked with an unlabeled oligonucleotide (Figure 4A, lane 4). To determine whether GRP mediates Ets1 transactivation, SK-N-SH cells were cotransfected with 5x GAL4-Luc with either wild-type GAL4-Ets1 (1–244) or mutant GAL4-Ets1 (T38A) plasmid. The cells were serum-starved for 24 hours and then stimulated with GRP (10-7 M) for 24 hours. Ets1 transactivation was enhanced by nearly 15-fold with GRP stimulation in SK-N-SH cells (Figure 4B). In contrast, the mutant Ets1 (T38A) was devoid of Ets1 transactivation activity. These data strongly suggest that the transactivation of Ets1 is induced in response to GRP treatment in SK-N-SH human neuroblastoma cells.
Figure 4.
GRP increases Ets1 DNA binding and transcription activity. (A) Nuclear protein was extracted from cells treated with GRP (10-7 M) for 4 hours. Nuclear protein (2.5 µg/lane) was added in binding reactions, and protein/DNA complexes were resolved in a 6% DNA retardation gel. (B) SK-N-SH cells were cotransfected with 5x GAL4-Luc plus GAL4-Ets1 (WT) or GAL4-Ets1 (T38A) (dominant-negative mutant vector). Cells were treated with GRP (10-7 M) for 24 hours after serum starvation. Ets luciferase activity was significantly increased after GRP treatment (data represent triplicate determinations; mean ± SEM; *P < .05 vs control vector).
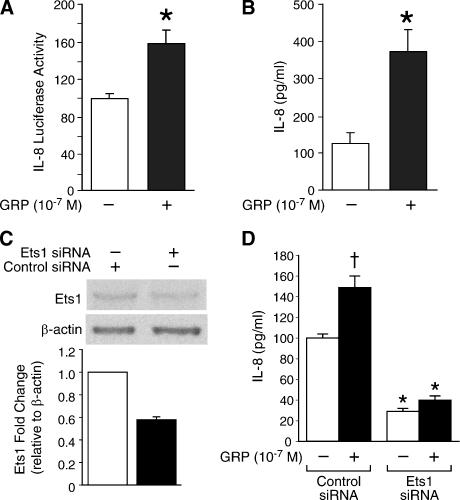
GRP Treatment Enhances IL-8 Luciferase Activity and Ets1 Regulates GRP-Induced IL-8 Secretion
We have found that Ets1 is transactivated by GRP stimulation and that Ets1 increases IL-8 promoter activity and IL-8 secretion in neuroblastoma cells. Next, we further determined whether GRP stimulates IL-8 transcription and secretion through the Ets1 transcription factor. SK-N-SH cells were transfected with IL-8 luciferase plasmid and then treated with GRP (10-7 M). GRP increased IL-8 promoter activity to > 58% when compared to control at 24 hours (Figure 5A). Moreover, GRP treatment also resulted in a nearly three-fold increase in IL-8 secretion (Figure 5B). To further validate the critical role of Ets1 during GRP-induced IL-8 upregulation, we next transfected SK-N-SH cells with Ets1 siRNA vector, which resulted in suppression of Ets1 protein levels by 50% (Figure 5C). Targeted Ets1 knockdown significantly attenuated GRP-stimulated IL-8 secretion, when compared to control cells transfected with control siRNA vector (Figure 5D). Interestingly, Ets1 siRNA transfection alone without GRP resulted in a significant decrease in IL-8 secretion (Figure 5D). Taken together, these results further demonstrate that the Ets1 transcription factor plays an important role in regulating IL-8 transcription and secretion in neuroblastoma cells.
Figure 5.
GRP increases IL-8 luciferase activity, and Ets1 regulates GRP-induced IL-8 secretion in SK-N-SH cells. (A) For IL-8 promoter activity assays, cells were seeded in 24-well plates, transfected with IL-8 luciferase plasmid DNA, and then treated with GRP (10-7 M). (B) For IL-8 secretion assay, cells in 12-well plates (1 x 105 cells/well) were treated with GRP (10-7 M) for 24 hours. The culture supernatants were collected, and IL-8 in the culture supernatant was measured by ELISA (data represent triplicate determinations; mean ± SEM; *P < .05 vs control). (C) Ets1 siRNA transfection yielded a significantly decreased Ets1 protein expression by Western blot analysis. (D) Ets1 knockdown using siRNA significantly decreased IL-8 secretion. In addition, GRP-stimulated increase in IL-8 secretion was markedly attenuated in SK-N-SH cells transfected with Ets1 siRNA vector when compared to control cells transfected with control siRNA vector (mean ± SEM; *P < .05 vs control siRNA vector; †P < .05 vs no GRP). All data represent triplicate determinations.
Discussion
We have previously shown that GRP acts as a mitogenic factor in neuroblastoma cells. Moreover, GRP-R overexpression downregulates the expression of the tumor-suppressor gene PTEN and promotes cell proliferation [20]. In this study, we found that GRP activates the Ets1 transcription factor and causes Ets1 nuclear localization. Activated Ets1 further induced proangiogenic factor IL-8 expression and secretion. This process may be an important mechanism contributing to aggressive tumor behavior as a result of enhanced angiogenesis in advanced-stage neuroblastomas.
Neuroblastoma tumor growth and metastasis depend on nutrients supplied through neovascularization. A high vascular index in neuroblastoma correlates with a poor overall prognosis in patients [3]. IL-8 is an inflammatory cytokine and potent angiogenic factor in vitro and in vivo. IL-8 induces neovascular capillary formation in many solid tumors [21].
In neuroblastoma cells, IL-8 is both expressed and secreted [8]. In addition, the overexpression of MYCN, a critical oncogene for neuroblastoma, has been shown to upregulate IL-8 expression and secretion [22]. Hypoxia induces the expression of IL-8 in vascular endothelial cells through the activation of NF-κB [23] and can be enhanced by the activation of the PI3-K/Akt and p38 MAPK pathways [24]. Hoffmann et al. [12] reported that c-Fos and Fos-related antigen-1, two inducible components of AP-1, counteractively regulate the endogenous IL-8 promoter in an IL-1-dependent manner. Recently, it has been revealed that MEF, a member of Ets family transcription factors, is a potent activator of IL-8 expression in hematopoietic cells [13]. Correspondingly, in this study, we also found that Ets1 expression significantly upregulates IL-8 expression and secretion in human neuroblastoma cells.
Ets1, a pro-oncogenic protein, is produced by a variety of solid tumors, including neuroblastomas, epithelial tumors, sarcomas, and astrocytomas. Ets1 expression is increased in invasive advanced-stage tumors and is an indicator of poor clinical prognosis [25]. Ets1 also promotes angiogenesis. It has recently been shown that there is a significant correlation between microvasculature and ets1 gene expression levels in uterine endometrial cancers [26]. In another study, constitutive Ets1 expression has been shown to stimulate angiogenesis and to induce an invasive phenotype [27]. Our current study demonstrated that Ets1 expression also correlates with tumor cellular differentiation, where increased expression of Ets1 protein was detected in more undifferentiated human neuroblastomas when compared to Ets1 protein levels in well-differentiated tumors with mature ganglion cells. However, a variable expression of Ets1 protein was noted in six human neuroblastoma cell lines irrespective of their histologic features (data not shown). More importantly, overexpression of Ets1 upregulated IL-8 transcription and secretion in neuroblastoma cells, strongly suggesting a correlation of Ets1 expression with angiogenesis in human neuroblastomas.
In SK-N-SH cells, we found that GRP increased the phosphorylation of Ets1 transcription factor, induced Ets1 nuclear localization, and increased Ets1 DNA-binding activity. Certainly, GRP-mediated activation of transcription factors is not limited to Ets1 alone. Bombesin, an analog of GRP, which acts as a mitogen, is known to induce the expression of the early response genes c-fos and c-jun, and also activates transcription factor Elk1 in prostate cancer cell lines [28]. In non-small cell lung cancer cells, GRP promotes cell proliferation through transactivation of EGF receptor and induction of its downstream MAPK pathway [29]. Previously, we have demonstrated the role of GRP as an autocrine growth factor for neuroblastoma cells [6]. Therefore, our current findings suggest that the mitogenic action of GRP may, in part, be mediated through the activation of the proangiogenic Ets1 transcription factor.
Moreover, our present study showed that GRP moderately upregulates the proangiogenic factor IL-8 both at the transcriptional level and on protein secretion. Similarly, Levine et al. [30] reported that bombesin stimulation of GRP-R resulted in the upregulation of IL-8 and VEGF gene expression through an NF-κB-dependent pathway in androgen-insensitive prostate cancer cells lines. Another group reported that bombesin-related peptides possess immunoregulatory activities because bombesin stimulated superoxide anion production and IL-8 release by peripheral monocytes, enhancing monocyte and macrophage activities and promoting local pulmonary defenses in chronic bronchitis [31]. These studies further support our findings demonstrating the role of GRP/GRP-R in angiogenesis and, potentially, in immune modulation in neuroblastomas. Tumor invasiveness is regulated by multiple factors. Recently, the coordinated expression of integrin subunits, matrix metalloproteinases (MMPs), angiogenic genes IL-8 and PEA3, and Ets-1 transcription factors has been detected in advanced-stage ovarian carcinoma [32]. It will be of significance to identify whether such mechanisms also exist in GRP-R-mediated neuroblastoma invasiveness. Our preliminary data indicate that GRP-R overexpression upregulates MMP-1 and MMP-3 and downregulates TIMP-1, a tissue inhibitor of metalloproteinase. GRP treatment increased MMP-2 and MMP-9 and decreased TIMP-1 protein levels (unpublished data).
Taken together, we conclude that GRP promotes angiogenesis in neuroblastomas, by activating the Ets1 transcription factor and its downstream target IL-8, to induce aggressive tumor behavior. Tumor vascularity correlates with an aggressive phenotype suggesting that antiangiogenic therapy may be a useful addition to current treatment strategies for high-risk advanced-stage neuroblastomas.
Acknowledgements
The authors thank Karen Martin for manuscript preparation, Lan Pang for technical assistance, and Tatsuo Uchida for statistical analysis.
Abbreviations
- GRP
gastrin-releasing peptide
- GRP-R
gastrin-releasing peptide receptor
- IL
interleukin
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by grants RO1 DK61470, RO1 DK48498, RO1 CA104748, and PO1 DK35608 from the National Institutes of Health.
References
- 1.Shusterman S, Maris JM. Prospects for therapeutic inhibition of neuroblastoma angiogenesis. Cancer Lett. 2005;228:171–179. doi: 10.1016/j.canlet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 2.Ribatti D, Marimpietri D, Pastorino F, Brignole C, Nico B, Vacca A, Ponzoni M. Angiogenesis in neuroblastoma. Ann NY Acad Sci. 2004;1028:133–142. doi: 10.1196/annals.1322.014. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D, Ponzoni M. Antiangiogenic strategies in neuroblastoma. Cancer Treat Rev. 2005;31:27–34. doi: 10.1016/j.ctrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Ribatti D, Vacca A, Nico B, De Falco G, Giuseppe Montaldo P, Ponzoni M. Angiogenesis and anti-angiogenesis in neuroblastoma. Eur J Cancer. 2002;38:750–757. doi: 10.1016/s0959-8049(01)00337-9. [DOI] [PubMed] [Google Scholar]
- 5.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Hu W, Kelly DR, Hellmich MR, Evers BM, Chung DH. Gastrin-releasing peptide is a growth factor for human neuroblastomas. Ann Surg. 2002;235:621–629. doi: 10.1097/00000658-200205000-00003. (discussion, 629–630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- 8.Ferrer FA, Pantschenko AG, Miller LJ, Anderson K, Grunnet M, McKenna PH, Kreutzer D. Angiogenesis and neuroblastomas: interleukin-8 and interleukin-8 receptor expression in human neuroblastoma. J Urol. 2000;164:1016–1020. doi: 10.1097/00005392-200009020-00024. [DOI] [PubMed] [Google Scholar]
- 9.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 10.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 11.Fibbe WE, Pruijt JF, Velders GA, Opdenakker G, van Kooyk Y, Figdor CG, Willemze R. Biology of IL-8-induced stem cell mobilization. Ann NY Acad Sci. 1999;872:71–82. doi: 10.1111/j.1749-6632.1999.tb08454.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann E, Thiefes A, Buhrow D, Dittrich-Breiholz O, Schneider H, Resch K, Kracht M. MEK1-dependent delayed expression of Fos-related antigen-1 counteracts c-Fos and p65 NF-kappaB - mediated interleukin-8 transcription in response to cytokines or growth factors. J Biol Chem. 2005;280:9706–9718. doi: 10.1074/jbc.M407071200. [DOI] [PubMed] [Google Scholar]
- 13.Hedvat CV, Yao J, Sokolic RA, Nimer SD. Myeloid ELF1-like factor is a potent activator of interleukin-8 expression in hematopoietic cells. J Biol Chem. 2004;279:6395–6400. doi: 10.1074/jbc.M307524200. [DOI] [PubMed] [Google Scholar]
- 14.Graves BJ, Petersen JM. Specificity within the ets family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 15.Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N. The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J Pathol. 2001;194:43–50. doi: 10.1002/path.844. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama T, Ito M, Ohtsuru A, Naito S, Sekine I. Expression of the ets-1 proto-oncogene in human colorectal carcinoma. Mod Pathol. 2001;14:415–422. doi: 10.1038/modpathol.3880328. [DOI] [PubMed] [Google Scholar]
- 17.Lelievre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- 18.Shimada H. Neuroblastoma. Pathology and biology. Acta Pathol Jpn. 1992;42:229–241. doi: 10.1111/j.1440-1827.1992.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao J, Kang J, Cree J, Evers BM, Chung DH. Gastrin-releasing peptide-induced down-regulation of tumor suppressor protein PTEN (phosphatase and tensin homolog deleted on chromosome ten) in neuroblastomas. Ann Surg. 2005;241:684–691. doi: 10.1097/01.sla.0000161173.47717.71. (discussion, 691–682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facchetti P, Prigione I, Ghiotto F, Tasso P, Garaventa A, Pistoia V. Functional and molecular characterization of tumour-infiltrating lymphocytes and clones thereof from a major-histocompatibilitycomplex-negative human tumour: neuroblastoma. Cancer Immunol Immunother. 1996;42:170–178. doi: 10.1007/s002620050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Chan HM, Li Z, Lin C, Nicholls J, Chen CF, Lee PY, Lui V, Bacher M, Tam PK. Upregulation of macrophage migration inhibitory factor contributes to induced N-myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene. 2004;23:4146–4154. doi: 10.1038/sj.onc.1207490. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Pathak PS, Fukumura D. Hypoxia-induced activation of p38 mitogen-activated protein kinase and phosphatidylinositol 3′-kinase signaling pathways contributes to expression of interleukin 8 in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:701–707. doi: 10.1158/1078-0432.ccr-0953-03. [DOI] [PubMed] [Google Scholar]
- 25.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto J, Aoki I, Toyoki H, Khatun S, Tamaya T. Clinical implications of expression of ETS-1 related to angiogenesis in uterine endometrial cancers. Ann Oncol. 2002;13:1605–1611. doi: 10.1093/annonc/mdf334. [DOI] [PubMed] [Google Scholar]
- 27.Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin beta3. J Cell Physiol. 1999;178:121–132. doi: 10.1002/(SICI)1097-4652(199902)178:2<121::AID-JCP1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Qu X, Weber HC. GRP receptor -mediated immediate early gene expression and transcription factor Elk-1 activation in prostate cancer cells. Regul Pept. 2002;109:141–148. doi: 10.1016/s0167-0115(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 29.Thomas SM, Grandis JR, Wentzel AL, Gooding WE, Lui VW, Siegfried JM. Gastrin-releasing peptide receptor mediates activation of the epidermal growth factor receptor in lung cancer cells. Neoplasia. 2005;7:426–431. doi: 10.1593/neo.04454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine L, Lucci JA, III, Pazdrak B, Cheng JZ, Guo YS, Townsend CM, Jr, Hellmich MR. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003;63:3495–3502. [PubMed] [Google Scholar]
- 31.Meloni F, Ballabio P, Bianchi L, Mangiarotti P, Grassi G, Bignamini A, Grassi GG. Bombesin enhances monocyte and macrophage activities: possible role in the modulation of local pulmonary defenses in chronic bronchitis. Respiration. 1996;63:28–34. doi: 10.1159/000196512. [DOI] [PubMed] [Google Scholar]
- 32.Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Risberg B, Ben-Baruch G, Reich R. Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer Metastasis Rev. 2003;22:103–115. doi: 10.1023/a:1022272204045. [DOI] [PubMed] [Google Scholar]