Abstract
DNA polymerase ι (pol ι) is a conserved Y family enzyme that is implicated in translesion DNA synthesis (TLS) but whose cellular functions remain uncertain. To test the hypothesis that pol ι performs TLS in cells, we compared UV-induced mutagenesis in primary fibroblasts derived from wild-type mice to mice lacking functional pol η, pol ι, or both. A deficiency in mouse DNA polymerase η (pol η) enhanced UV-induced Hprt mutant frequencies. This enhanced UV-induced mutagenesis and UV-induced mutagenesis in wild-type cells were strongly diminished in cells deficient in pol ι, indicating that pol ι participates in the bypass of UV photoproducts in cells. Moreover, a clear strand bias among UV-induced base substitutions was observed in wild-type cells that was diminished in pol η- and pol ι-deficient mouse cells and abolished in cells deficient in both enzymes. These data suggest that these enzymes bypass UV photoproducts in an asymmetric manner. To determine whether pol ι status affects cancer susceptibility, we compared the UV-induced skin cancer susceptibility of wild-type mice to mice lacking functional pol η, pol ι, or both. Although pol ι deficiency alone had no effect, UV-induced skin tumors in pol η-deficient mice developed 4 weeks earlier in mice concomitantly deficient in pol ι. Collectively, these data reveal functions for pol ι in bypassing UV photoproducts and in delaying the onset of UV-induced skin cancer.
Keywords: translesion synthesis, UV mutagenesis, Y family polymerase, polymerase eta
DNA polymerase ι (pol ι) is a member of the evolutionarily conserved Y family of DNA polymerases that includes pol η (1). Y family enzymes participate in bypassing lesions in DNA that impede replication fork progression (reviewed in refs. 2–5). The most compelling evidence for a role in translesion synthesis (TLS) is with pol η. pol η can efficiently bypass a cis-syn thymine–thymine dimer (6, 7), it localizes at replication foci after UV irradiation of mammalian cells (8), and its inactivation in XPV patients (9, 10) increases UV light-induced mutagenesis and skin cancer (reviewed in refs. 4 and 11). Increased UV-induced mutagenesis associated with pol η deficiency indicates that mutagenic bypass of UV photoproducts can be catalyzed by other polymerases. One candidate is pol ζ (2), which can bypass a thymine–thymine dimer in vitro (12) and is required for a large proportion of UV mutagenesis in vivo (2, 13, 14). Another candidate is pol ι, which physically interacts with pol η and colocalizes with pol η at replication foci after UV irradiation (8).
Two hypotheses have been put forth regarding the outcome of putative UV photoproduct bypass by pol ι. One suggests that bypass should be mutagenic because of pol ι-catalyzed misinsertion of nucleotides opposite photoproducts (15, 16). pol ι is renowned for high rates of misinsertion of nucleotides opposite template pyrimidines during DNA synthesis in vitro (reviewed in ref. 3). However, a study of UV-induced mutagenesis in cultured 293T cells in which pol ι expression was decreased by using siRNA concluded that pol ι has no significant role in UV lesion bypass and mutagenesis (17). A second hypothesis is that pol ι bypass could be antimutagenic for UV-induced C to T transition mutations. This antimutagenic hypothesis derives from a pol ι preference for inserting dGMP opposite template T, leading to the suggestion (18, 19) that synthesis by pol ι could be antimutagenic by insertion of dGMP opposite lesions resulting from deamination of cystosine. Indeed, cytosines in dipyrimidine photoproducts readily deaminate (see refs. 20 and 21 and refs. therein), and pol ι readily inserts dGMP opposite template uracil in cyclobutane pyrimidine dimers (21).
One approach to investigate the function of TLS polymerases in DNA damage-induced carcinogenesis and mutagenesis is to examine the consequences of inactivating these polymerases in mice. Mice inactivated for pol η are viable and fertile and have increased susceptibility to UV light-induced skin cancer (22), altered angiogenesis early in neoplastic transformation (23), and altered somatic hypermutation (SHM) specificity in Ig genes (24, 25). This contrasts with a 129 mouse strain that contains a spontaneous nonsense mutation in the Polι gene that truncates pol ι at Ser-27 (26). These pol ι-deficient mice do not exhibit obvious spontaneous phenotypes (26). Moreover, the 129 mice (26) and a pol ι-deficient strain derived from them (27) exhibit normal SHM specificity and have normal class switch recombination. To our knowledge, these pol ι-deficient mice have not been reported to exhibit defects in responses to DNA-damaging agents. Here we report the effects of inactivating pol ι on UV light-induced mutagenesis and skin cancer. Results with primary fibroblasts from pol ι-deficient mice indicate that pol ι does play a role in bypass of UV photoproducts that is biased for one strand of DNA. An initial study of double-knockout mice indicates that, when pol η is inactivated, pol ι functions in a manner that delays onset of skin cancer.
Results
Generation of Mice and Primary Fibroblasts.
Mice with homozygous deficiencies in pol η and pol ι were generated by mating F2 Polη+/−/ι−/− mice. The progeny included 23 Polη+/+/ι−/− mice, 48 Polη +/−/ι−/− mice, and 30 Polη−/−/ι−/− mice, indicating that mice deficient in both pol η and pol ι develop normally and are viable. These mice were used to examine whether pol ι has a role in response to irradiation with UV light. While experiments were underway to examine the susceptibility of the mice to UV light-induced skin cancer (see below), primary fibroblasts were isolated from ear biopsies of wild-type mice and mice deficient in pol η only, pol ι only, and both pol η and pol ι, and these were examined for UV light-induced cytotoxicity and mutagenesis.
Survival of Primary Mouse Fibroblasts After UV Irradiation.
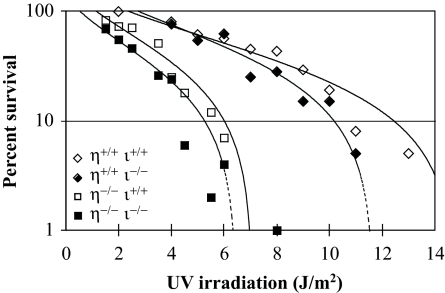
Cells deficient in pol η alone were moderately sensitive to killing by UV irradiation (D37, 2.8 J/m2) in comparison to wild-type cells (D37, ≈8 J/m2; Fig. 1). In either the pol η-deficient or -proficient background, cells deficient in pol ι were more sensitive to UV-induced cytotoxicity (Fig. 1). Although the differences are small, they are statistically significant as determined by polynomial regression analysis (see legend to Fig. 1).
Fig. 1.
Survival of primary mouse fibroblasts after UV irradiation. The influence of mouse genotype on survival of primary dermal fibroblasts after irradiation with UV254 nm was measured by colony-forming ability, as described in Methods. The fluence required to reduce the survival of Polη+/+/ι+/+ cells to 37% of the unirradiated control (D37) was 8 J/m2. This was reduced to ≈2.8 J/m2 in the cells derived from Polη−/−/ι+/+. In both Polη+/+ and Polη−/− backgrounds, disruption of Polι slightly reduced UV survival. The significance of the reduced survival of pol ι-deficient cells was determined by polynomial regression analysis (53), with the best fit provided by cubic polynomials. This analysis revealed that the percent survival for Polη+/+/ι−/− cells was significantly lower than for Polη+/+/ι+/+ cells (P = 0.018), and that the percent survival for Polη−/−/ι−/− cells was significantly lower than for Polη−/−/ι+/+ cells (P = 0.001).
UV Light-Induced Mutagenesis at the Hprt Locus in Primary Mouse Fibroblasts.
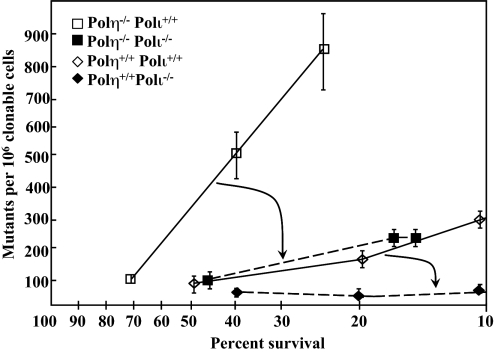
Compared with wild-type fibroblasts (Fig. 2, open diamonds), a deficiency in mouse pol η alone resulted in increased UV irradiation-induced mutagenesis at the endogenous Hprt locus (Fig. 2, open squares). Interestingly, in pol η-deficient cells in which pol ι was also deficient, the mutant frequency was also reduced (Fig. 2, closed squares), implicating pol ι in mutagenic TLS when pol η is absent. Even when pol η is proficient, pol ι deficiency reduced UV-induced mutagenesis (compare open to closed diamonds in Fig. 2), again implicating mouse pol ι in bypass of UV photoproducts. That the UV-induced mutant frequency in cells deficient in both pol η and pol ι (filled boxes) is higher than the spontaneous mutant frequency (≈10−5) indicates that a third polymerase performs mutagenic bypass of photoproducts.
Fig. 2.
Frequency of TG-resistant (TGr) clones as a function of survival after UV irradiation. Cells were plated on three 150-mm-diameter dishes at a density of 104 cells per dish to determine mutant frequency or at cloning density to determine survival. After attachment, plates were irradiated with UV fluences estimated from the data in Fig. 1 to yield 20–40% survival. The actual survival in the mutagenesis experiments was determined as in Fig. 1 and plotted on the x axis. The corresponding mutant frequency at each survival is plotted on the y axis. Each point represents the mean of three independent dishes at the indicated survival, ±1 SD. Mutant frequency is defined as the number of TGr clones per million clonable cells. Each data point represents independent experiments in which 2–4 × 106 surviving cells were selected after UV irradiation and an 8- to 9-day expression period. The data have been corrected for cloning efficiency on the day of selection, and the spontaneous background mutant frequency (1 × 10−5) has been subtracted. The arrows indicate the reduction in mutant frequency when Polι is disrupted in the pol η-deficient background (larger arrow) and in the pol η-proficient background (smaller arrow).
Specificity of UV-Induced Mutagenesis.
To discover the types and locations of pol ι-dependent UV-induced mutations, we sequenced independent UV-induced Hprt mutants derived from cells of each of the four genotypes. The results are summarized in Table 1 and presented in detail as Tables 2–5, which are published as supporting information on the PNAS web site. In all four instances, we analyzed mutant clones for exposures that resulted in ≈37% survival for that genotype.
Table 1.
UV-induced hprt mutation frequencies in mouse primary fibroblasts
|
Polη+/+Polι+/+ frequency (×10−6) |
Polη−/−Polι+/+ frequency (×10−6) |
Polη+/+Polι−/− frequency (×10−6) |
Polη−/−Polι−/− frequency (×10−6) |
|
|---|---|---|---|---|
| Mutation freq.* | 80 [36]† | 550 [48]† | 32 [19]† | 120 [20]† |
| Frameshifts | 4 (2)‡ | 23 (2) | ≤2 (0) | 6 (1) |
| Large deletions§ | 4 (2) | 92 (8) | 2 (1) | 29 (5) |
| Large insertions | 2 (1) | ≤11 (0) | ≤2 (0) | ≤6 (0) |
| Base substitutions at dipyrimidine sites | 60 (27) | 390 (34) | 24 (14) | 87 (15)¶ |
| Tandems | ≤2 (0) | 23 (2) | 5 (3) | 6 (1) |
| Transitions | 31 (14) | 180 (16) | 8 (5) | 41 (7) |
| C·G → T·A | 29 (13) | 100 (9) | 8 (5) | 23 (4)¶ |
| T·A → C·G | 2 (1) | 80 (7) | ≤2 (0) | 17 (3)¶ |
| Transversions | 29 (13) | 180 (16) | 10 (6) | 41 (7) |
| C·G → A·T | 4 (2) | 100 (9) | 2 (1) | 23 (4)¶ |
| C·G → G·C | ≤2 (0) | 11 (1) | ≤2 (0) | ≤6 (0) |
| T·A → A·T | 13 (6) | 57 (5) | 8 (5) | 17 (3)¶ |
| T·A → G·C | 11 (5) | 11 (1) | ≤2 (0) | ≤6 (0) |
Frequency (Freq.) measurements were determined for at least six independent assays and using primary fibroblasts isolated from at least two independent mice.
*Mutation frequencies were determined at UV exposures resulting in 37% cell survival.
†Numbers in brackets are the number of independent mutants sequenced (see Tables 2–5).
‡Numbers in parentheses are the number of independent mutations observed (see Tables 2–5).
§Large deletions are primarily deletions of exons (see Tables 2–5).
¶Three mutants contained multiple mutations separated by >100 nucleotides (see Table 5).
Wild-type cells.
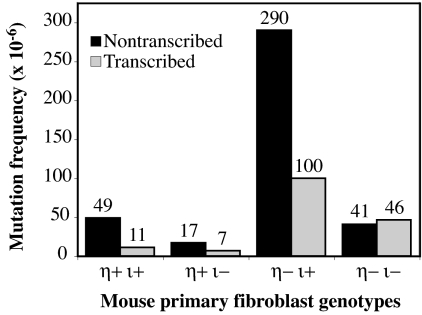
Among 36 Hprt mutant clones from wild-type cells, 31 base-pair substitutions were found (Tables 1 and 2). Twenty-seven substitutions occurred at dipyrimidines, indicating that dipyrimidine photoproducts are responsible for the majority of the UV-induced mutations. Interestingly, 22 of the 27 substitutions are inferred to result from bypass of a dipyrimidine photoproduct on the nontranscribed strand (Table 2). This represents a 4.5-fold bias in favor of mutations templated at dipyrimidines on the nontranscribed strand compared with the transcribed strand (Fig. 3, 49 × 10−6 vs. 11 × 10−6, P < 0.0001).
Fig. 3.
Strand specificity of UV-induced mutagenesis. The strand containing the dipyrimidine sequence was determined in the case of each targeted base substitution. The proportion of mutations that arose from putative photoproducts in either strand was multiplied by the mutant frequency induced at D37 for each of the four genotypes as presented in Fig. 2.
pol η-deficient cells.
A deficiency in mouse pol η alone resulted in an ≈7-fold increase in induced mutant frequency (Table 1; compare 80 × 10−6 to 550 × 10−6). Sequence analysis of UV-induced Hprt mutants in pol η-deficient fibroblasts (Tables 1 and 3) revealed increased frequencies for several types of single base substitutions (up to 40-fold for T-A to C-G substitutions), for tandem double-base substitutions (>10-fold), and for exon deletions (20-fold). The exon deletions could result from splicing abnormalities caused by mutations in splice donor or acceptor sites. Most substitutions were at dipyrimidines (Table 3). However, the bias in favor of mutations templated at dipyrimidines in the nontranscribed strand was reduced from 4.5-fold in wild-type cells to 2.9-fold in pol η-deficient cells (Fig. 3; 290 × 10−6 vs. 100 × 10−6), consistent with an earlier study using xeroderma pigmentosum (XP) variant cells (28) and the idea that pol η is acting to preferentially bypass photoproducts in the transcribed strand. Also, of note is the high proportion of CG to AT tranversions, 78% (7 of 9) of which arose from dipyrimidines on the transcribed strand.
In the present study of asynchronously growing mouse cells lacking pol η, similar frequencies and proportions of transitions and transversions were observed after UV irradiation, and the frequency of C to T transitions was 100 × 10−6 (Table 1). These data are similar to those reported in a previous study of the base substitution specificity of UV-induced mutagenesis at the HPRT locus in XP variant cells deficient in pol η (28). In that study, when human cells were irradiated at the beginning of S phase, UV-induced substitutions included 13 transitions and 17 transversions, and we calculated that the frequency of C to T transitions was 130 × 10−6. However, when the XP variant cells were irradiated in the G1 phase to allow time for repair before replication, transversions exceeded transitions by 11:4, and we calculated that the frequency of UV-induced C to T transitions was only 18 × 10−6. The differences between our study in mouse cells and human XP variant cells irradiated in the G1 could partly be related to the temporal relationship between repair and replication and/or the amount of time available for deamination of cytosine-containing photoproducts, especially because rodent cells have a reduced level of global nucleotide excision repair compared with human cells.
pol ι-deficient cells.
Fibroblasts deficient in pol ι have UV-induced total mutant frequencies and base substitution frequencies that are 2-fold lower than for wild-type cells (Table 1, compare columns 1 and 3). These differences are significant (P < 0.0001). Among 18 base substitutions, 14 were at dipyrimidines (Table 4). Notably, the dipyrimidine was on the nontranscribed strand for 10 of these, such that the nontranscribed to transcribed strand bias was 2.4-fold (Fig. 3, 17 × 10−6 vs. 7 × 10−6, P = 0.064). This is less than the 4.5-fold bias observed in wild-type cells, suggesting that the strand bias for UV-induced mutagenesis in wild-type cells partly depends on pol ι.
Cells deficient in both pol η and pol ι.
Fibroblasts deficient in pol η and pol ι (Table 5) had UV-induced mutant frequencies (Table 1, column 4) that, in comparison to cells deficient in pol η alone (column 2), were reduced for all mutations and for total substitutions, transitions, transversions, tandem double-base substitutions, and exon deletions. These 4- to 5-fold differences are significant (P ≤ 0.05). Among 15 substitutions at dipyrimidines, seven are inferred to result from bypass of a lesion on the nontranscribed strand, and eight are inferred to result from bypass of a lesion on the transcribed strand (Table 5). That deficiency in both pol η and pol ι eliminated the strand bias (Fig. 3; 41 × 10−6 vs. 46 × 10−6) suggests this bias depends on both polymerases. Also note that the UV-induced mutant frequency of fibroblasts deficient in both pol η and pol ι (120 × 10−6, Table 1) is much higher than the spontaneous mutant frequency in unirradiated cells (1 × 10−5), indicating that at least one other polymerase performs mutagenic TLS.
Susceptibility of pol η/ι Double-Knockout Mice to UV-Induced Skin Carcinoma.
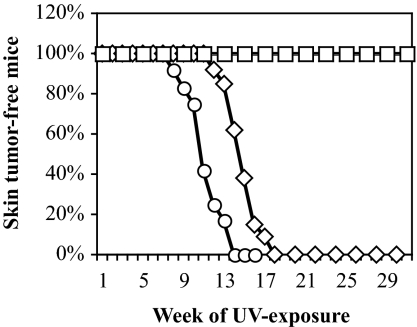
To determine the susceptibility of Polη−/−/ι−/− mutant mice to UV light-induced skin carcinoma, sets of littermates were shaved on part of their backs once per week, UV-irradiated three times per week at 3.75 kJ/m2, and examined for skin tumors each week. Irradiation was for 20 weeks or until the first skin tumor was observed. Twelve Polη−/−/ι−/− mutant mice, 13 Polη+/−/ι−/− mutant mice, and nine Polη+/+/ι−/− mutant mice were treated and examined, and the results were compared with those reported earlier for wild-type and Polη−/− mutant mice (22). Similar to results with wild-type mice (Fig. 4, open squares; from ref. 22), none of the 13 Polη+/−/ι−/− mutant mice or the nine Polη+/+/ι−/− mutant mice developed skin tumors over the 20-week course of irradiation (data not shown). Similar to results with the Polη−/− mutant mice (22), the irradiated ears of the Polη−/−/ι−/− mutant mice became darker, curved, and atrophied, whereas littermate controls (e.g., Polη+/+/ι−/− mice) did not show such abnormalities or develop skin tumors, even after 30 weeks of exposure. More importantly, UV-induced skin tumors developed in the double-homozygous mice starting at week 8 of irradiation, and by week 13, all 12 mice had developed skin tumors (Fig. 4, open circles). This time to tumor formation is earlier than for Polη−/− knockout mice, the first of which developed UV-induced skin tumors at week 12 of irradiation and the last at week 18 (Fig. 4, open diamonds; reproduced from ref. 22). This pol ι-dependent difference in time to skin tumor formation is significant, with low P values as determined by three different statistical tests, a log rank test (P = <0.0001, ref. 29), a t test (P = <0.0001, ref. 30), and a Mann–Whitney test (P = 0.0002, ref. 31). Histological analysis of skin tumors (data not shown) revealed phenotypes in double-knockout mice that were indistinguishable from those of the Polη−/− single knockouts, i.e., development from squamous cell carcinoma in situ to invasive carcinomas. Thus, loss of pol ι increases the susceptibility of pol η-deficient mice to UV-induced skin cancer, with no detectable change in the tumor developmental pathway.
Fig. 4.
UV light-induced skin cancer in mice. Mice were shaved once per week and irradiated three times per week with 3.75 kJ/m2 for 20 weeks or until the first skin tumor arose. Mice were inspected weekly for the development of skin tumors. Results with 12 Polη−/−/ι−/− mice (open circles) are compared with results reported (22) for 14 wild-type mice (open squares) and 12 homozygous Polη knockout mice (open diamonds), eight of which were Polι+/−, and four of which were Polι+/−. We also irradiated 13 Polη+/−/ι−/− mice and nine Polη+/+/ι−/− mice, none of which developed skin tumors after 20 weeks of irradiation (not plotted). In fact, the latter mice did not develop skin tumors after >41 weeks.
Discussion
This study of UV-induced mutagenesis and carcinogenesis in mice deficient in either polη, polι, or both polymerases provides insights into the biological function of pol ι and has several implications regarding TLS and the mutagenic and carcinogenic effects of UV radiation.
pol ι Participates in Translesion DNA Synthesis.
As expected based on results with human cells (28, 32, 33) and mouse embryonic fibroblasts (22), primary fibroblasts deficient in mouse pol η alone are moderately sensitive to killing by UV irradiation compared with wild-type cells (Fig. 1). Also as expected based on results with XPV cells lacking pol η (28, 32), a deficiency in mouse pol η strongly enhances UV light-induced mutagenesis at the endogenous Hprt locus (Fig. 2). This is the starting point for the observation that UV mutagenesis in pol η-deficient cells is suppressed by inactivation of pol ι (Fig. 2, Table 1). This suggests that pol ι participates in mutagenic bypass of UV photoproducts when pol η is deficient. That pol ι deficiency also suppresses UV-induced mutagenesis in pol η-proficient cells (Fig. 2, Table 1) suggests that TLS is a normal function of pol ι, not only a replacement function when another TLS enzyme is missing.
The interpretation that pol ι can conduct mutagenic photoproduct bypass in mouse cells is consistent with earlier biochemical studies suggesting that pol ι contributes to enhanced UV mutagenesis in pol η-deficient XPV cells (16). Our results differ from a recent study in which pol ι expression in cultured 293T cells was decreased by using siRNA (17). In that study, decreased pol ι expression had no effect on UV-induced mutagenesis in the supF gene present in an extrachromosomal shuttle vector, leading to the conclusion that pol ι has no significant role in UV lesion bypass and mutagenesis. Experimental differences between that study and ours could account for the different conclusions, including incomplete silencing of pol ι in 293T cells or use of a gene in a shuttle vector vs. an endogenous chromosomal gene to monitor mutagenesis.
The extent to which pol ι contributes to bypass of UV light induced lesions may partly depend on the type of photoproduct (for review, see refs. 3 and 4). Although pol η bypasses cis-syn thymine–thymine dimers, it cannot bypass or insert nucleotides opposite the two distorted bases of a 6-4 photoproduct. In contrast, pol ι inserts nucleotides opposite 6-4 photoproducts. Because pol ι does not extend the resulting primer termini, other polymerases, e.g., pol ζ or pol κ, have been suggested to perform that task. Thus pol ι's role may sometimes be subservient to pol η, perhaps explaining why pol ι's effect on UV mutagenesis is ≈2-fold when pol η is present (Table 1). Perhaps pol ι predominantly inserts nucleotides opposite photoproducts not efficiently bypassed by pol η. Although that could occur at the replication fork, it may also possibly occur during filling of gaps left in the DNA after the fork has moved on (e.g., see ref. 34 and refs. therein). However, when pol η is missing, pol ι may assume a somewhat larger role in bypassing UV photoproducts, consistent with a 5-fold mutagenic effect when pol η is absent (Table 1). Moreover, the details of TLS by pol ι could differ in the presence or absence of pol η, because the two enzymes physically interact, and because pol ι localization to foci in UV-irradiated human cells is reduced in pol η-deficient cells (8). These ideas and the identity of the photoproducts responsible for mutagenesis could be tested by combining the pol η and pol ι defects studied here with mice defective in global and transcription-coupled nucleotide excision repair or with mice that express photolyases that selectively remove cyclobutane pyrimidine dimers or 6-4 photoproducts (35).
The specificity in Table 1 implies that pol ι contributes to multiple types of UV-induced base substitutions. The pol ι-dependent substitution specificity in Table 1 is generally consistent with the insertion specificity of pol ι, which preferentially misinserts dNTPs opposite template pyrimidines (reviewed in ref. 3). The exception may be the high rate of C to T transitions that could result from incorporation of dAMP opposite a damaged pyrimidine (Table 1). UV-induced C to T transitions may primarily result from “correct” insertion of dAMP opposite uracil that results from deamination of cystosine in photoproducts (see refs. 20 and 21 and refs. therein). Based on that idea, the ability of pol ι to preferentially insert dGMP opposite U could theoretically be antimutagenic for cytosine deamination in general (18) and/or during TLS of photoproducts (21). That hypothesis is not supported by our observation here that pol ι promotes rather than suppresses UV-dependent mutagenesis for C to T transitions (Table 1).
pol η and pol ι Participate in Strand-Specific DNA Transactions.
In wild-type cells or cells deficient in pol η alone or pol ι alone, there is a bias for UV-induced base substitutions at dipyrimidines on the nontranscribed DNA strand (Fig. 3). This bias may reflect removal of photoproducts from the transcribed strand by transcription-coupled nucleotide excision repair, because such repair is known to alter the mutation spectrum (33, 36). Relative to wild-type cells, the strand bias is slightly reduced when pol η or pol ι is deficient and is eliminated when both enzymes are deficient (Fig. 3). This suggests that both polymerases contribute to modulating mutagenic bypass of UV photoproducts in a strand-specific manner. The strand biases could be related to differences in leading vs. lagging strand replication enzymology (see below), transcription, or perhaps a more prominent role in bypassing a subset of photoproducts that remain in the nontranscribed strand for a longer time.
Strand Bias for UV-Induced C-G to A-T Transversions.
The exception to the nontranscribed strand bias is that the majority of C-G to A-T substitutions are inferred to result from lesions in the transcribed strand. This observation in mouse cells is similar to results in XP variant cells (28). In human cells, a chromosomal origin of replication is located 3′ to exon 1 of HPRT (37), implying that the transcribed strand of HPRT is replicated as the leading strand template. That interpretation is consistent with studies of replication in extracts of XP variant cells, which generates a high proportion of C-G to A-T substitutions arising from replication of photoproducts on the leading strand template (38). By extrapolation, the C-G to A-T substitutions in the present study may result from leading strand replication, leading to the further speculation that the nontranscribed strand bias discussed above could reflect TLS during replication of the lagging strand template. In addition to CPDs and 6-4 photoproducts, UV irradiation also generates oxidative damage to DNA. Thus oxidative lesions like 8-oxo-guanine may contribute to the G-C to T-A transversions observed in irradiated fibroblasts and possibly to skin cancer. However, this may be unlikely, because monobasic forms of DNA damage induced by the wavelengths used in the present study occur at <1% of the frequency of dipyrimidine photoproducts (39). Interestingly, the C-G to A-T transversions on the transcribed strand largely originate when pol η is deficient, suggesting they are made by a different TLS polymerase such as pol ι (in pol η-deficient cells; Table 4) or perhaps pol ζ (in doubly deficient cells; Table 5).
At Least One Other Polymerase Participates in Bypass of UV Photoproducts.
The observation that the UV-induced mutant frequency in cells deficient in both pol η and pol ι (Table 1) is much higher than the spontaneous mutant frequency of unirradiated cells indicates that at least one other polymerase performs mutagenic TLS. One candidate is pol ζ (reviewed in ref. 4). Notably, yeast pol ζ has recently been shown to efficiently bypass UV photoproducts when assisted by accessory proteins (12), and it copies undamaged templates with low fidelity (40).
pol ι Has a Function in Delaying Onset of UV Light-Induced Skin Cancer.
There are well known correlations between increased mutagenesis and increased carcinogenesis, and there is considerable evidence to support the hypothesis that a mutator phenotype promotes multistage carcinogenesis (41). Thus the observation that UV light-induced skin cancer is suppressed by pol ι (Fig. 4) was unexpected, even though pol ι appears to contribute to mutagenic bypass of photoproducts (Fig. 2, Table 1). Several hypotheses can be considered. It could simply be that mutagenesis at the Hprt locus in primary fibroblasts does not reflect rate-limiting mutations in the cell types and/or genes most relevant to development of skin tumors in mice. Against this are similar measurements of mutagenesis at the HPRT locus in pol η-deficient XPV fibroblasts that do demonstrate elevated UV-induced mutant frequencies that correlate with elevated susceptibility to skin cancer. It could be that the function of pol ι that suppresses skin cancer is simply to bypass lesions, regardless of whether this is performed in an accurate or mutagenic fashion. Alternatively, pol ι could modulate cancer susceptibility by a function other than TLS. Recent studies have reported that polymerases implicated in TLS also participate in recombination (42, 43), in nucleotide excision repair (44), and in checkpoint response to UV irradiation (45). If pol ι were to have similar roles in the absence of pol η, then loss of function could increase susceptibility to skin cancer. In fact, loss of either TLS or checkpoint control because of pol ι deficiency might logically be anticipated to result in increased UV-induced cytotoxicity, consistent with the slightly reduced survival observed in the present study (Fig. 1). That the cytotoxic effect is not stronger could indicate that some photoproducts are initially tolerated and later bypassed by a different polymerase to yield delayed mutagenesis (46, 47). Another possibility is that pol ι may play an important role in development of normal immunity, such that pol ι deficiency would compromise immune suppression of skin cancer and lead to increased susceptibility. Against this possibility are reports that class switch recombination and somatic hypermutation of Ig genes appear to be normal in 129-derived strains of mice (26, 27). Finally, it is formally possible that some cells in double-knockout mice may retain pol ι activity. As mentioned in the Introduction, 129 mice are homozygous for a spontaneous C to A mutation in exon 2 that creates a nonsense codon in the Polι gene at Ser-27, such that the protein should be truncated at amino acid 26 (26). Indeed, no pol ι protein was detected by Western blotting of testis extracts of 129 mice using an antibody raised against the COOH terminus of murine pol ι that recognizes full-length or misspliced variants of pol ι (26). However, a recent study (48) has reported that crude extracts of brain cells from 129/J mice (but not extracts of other 129/J cells) have the ability to insert dGMP opposite template T in an oligonucleotide primer template. The authors claim this misinsertion activity is due to pol ι rather than any of the other DNA polymerases that can insert dGMP opposite T, and they further speculate that brain cells of 129 mice contain alternatively spliced pol ι that retains catalytic activity.
The observation that pol ι delays onset of skin cancer in pol η-deficient mice (Fig. 4) is consistent with other studies suggesting a role for mouse pol ι in tumor suppression. The mouse Polι gene is on chromosome 18 and within the Par2 (pulmonary adenoma resistance 2) locus that is a major determinant of susceptibility to urethane-induced pulmonary adenomas. Interestingly, two strains of mice with differing lung tumor susceptibility differ in Polι gene status by 25 nucleotides and 10 amino acids and the two enzymes have altered substrate specificity, suggesting that pol ι is a modifier of lung tumorigenesis (49). More recently, the defective Polι allele in the 129×1/Sv mouse strain that harbors the nonsense mutation in the coding sequence has been associated with susceptibility to urethane-induced lung tumors (50).
Methods
Generation of Double-Knockout Mice.
In an earlier study (22), an ES cell line derived from pol ι-deficient 129 mice (26) was used to generate pol η knockout mice. In that study (22), chimeric mice were bred with B6 mice to produce F1 Polη heterozygotes that were then intercrossed to generate the F2 mice. The present study began by PCR-genotyping 595 F2 mice. Twelve were Polη+/−/ι−/−, and these were intercrossed to generate the double-homozygous mice studied here.
Generation and Growth of Primary Ear Fibroblasts.
Primary fibroblasts derived from ear punches as described (14) were grown at 37°C under hypoxic conditions (2–3% O2, 5% CO2) previously reported to increase the number of population doublings and lengthen the time before senescense of primary murine cells (51). Nitrogen gas was used to sustain hypoxic conditions in the incubators using an oxygen control module (BioSpherix, Redfield, NY; PROOX model 110). Cells were maintained in MEM-α (Cambrex Bioscience, Walkersville, MD) supplemented with 10% FBS (HyClone, Logan, UT), 2 mM glutamine, nonessential amino acids (Mediatech, Herndon, VA), penicillin (100 units/ml), and streptomycin (100 μg/ml) for all assays with the exception of the thioguanine (TG) screening, which used DMEM (Mediatech).
Cytotoxic and Mutagenic Effects of UV Irradiation.
Fibroblasts were assayed for cytotoxic and mutagenic responses to UV254 nm radiation at early passages (six to eight population doublings) after primary cultures were established. The UV source was a Spectroline germicidal lamp that emits short-wave UV with a peak emission at 254 nm. The energy emitted by the light was quantified by using a radiometer (model IL 1700; International Light, Peabody, MA) and a sensor fitted with a 254-nm filter and W diffuser. The height of the light was adjusted to deliver 0.1–0.2 J/m2/sec at the level of the cells. A series of independent populations (1.5 × 106 cells each, plated on 150-mm-diameter plates) were treated with UV254 nm at fluences of 0–12 J/m2 for Polη+/+ cells or 0–6 J/m2 for Polη−/− cells. Before UV exposure, the culture medium was aspirated, and the cells were washed with PBS (pH 7.4). Sufficient dishes were used to ensure at least 1.0 × 106 surviving cells. Each dish was allowed 3–5 days of growth before trypsinization and passage of 1.5 × 106 cells. After 8–9 days of postirradiation expression, 0.5–1.0 × 106 cells were selected for 6-TG resistance, as described (14). Cytotoxic response to radiation was established by plating cells at cloning density and measuring colony-forming ability. The media of cells exposed at cloning density were replaced with fresh medium at 24 h and 7 days after irradiation and stained at ≈14 days. Colony-forming ability was also determined at the time of TGr selection by plating the cells at cloning density in nonselective medium. This value was used to correct the observed frequency of mutants. After 14–20 days, TGr resistant clones were isolated in RNase-free PBS for Hprt coding region amplification. After colony isolation, plates were stained, and mutant frequency was determined, defined as the number of observed TGr clones per 106 clonable cells (corrected for cloning efficiency). The significance of differences in frequencies between polymerase genotypes was calculated by using Fisher's exact test (52).
Amplification and Sequencing of Hprt cDNA.
Isolation of TGr clones, reverse transcriptase-PCR of the coding region of the Hprt gene, and sequence analysis of the PCR products were done as described (14). The sequence of both DNA strands was determined by using dRhodamine dye terminator chemistry (Applied Biosystems, Foster City, CA).
Irradiation of Mice.
Mice were irradiated with UVB light and characterized as described (22). The UV source was a bank of two UVB lamps (Blak-Ray lamp Model XX-15M; UV Products, Upland, CA) that emit wavelengths in the 280- to 370-nm range, with a peak at 302 nm. UVB flux was measured by a UVX digital radiometer (Model UVX-31; UV Products).
Supplementary Material
Acknowledgments
We thank Marilyn Diaz and Bennett Van Houten for thoughtful comments on the manuscript, Tom J. Burke for assistance in amplifying Hprt cDNA from mutant clones, and Dinh Nguyen for assistance in sequence analysis of Hprt mutants. This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (to T.A.K.), by NIH Grants CA112197 and CA112664 (to W.G.M.), by a grant from the Kentucky Lung Cancer Research Board (to C.A.D.), and by NIH Grants CA84301 and EH 11040 (to R.K.).
Abbreviations
- pol ι
DNA polymerase ι
- TLS
translesion synthesis
- TG
thioguanine
- TGr
TG resistant
- XP
xeroderma pigmentosum
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, et al. Mol Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence CW. Adv Protein Chem. 2004;69:167–203. doi: 10.1016/S0065-3233(04)69006-1. [DOI] [PubMed] [Google Scholar]
- 3.Vaisman A, Lehmann AR, Woodgate R. Adv Protein Chem. 2004;69:205–228. doi: 10.1016/S0065-3233(04)69007-3. [DOI] [PubMed] [Google Scholar]
- 4.Prakash S, Johnson RE, Prakash L. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, DC: Am Soc Microbiol; 2006. [Google Scholar]
- 6.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RE, Prakash S, Prakash L. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 8.Kannouche P, Fernâandez de Henestrosa AR, Coull B, Vidal AE, Gray C, Zicha D, Woodgate R, Lehmann AR. EMBO J. 2003;22:1223–1233. doi: 10.1093/emboj/cdf618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RE, Kondratick CM, Prakash S, Prakash L. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 11.Cleaver JE. Nat Rev Cancer. 2005;5:564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- 12.Garg P, Stith CM, Majka J, Burgers PM. J Biol Chem. 2005;280:23446–23450. doi: 10.1074/jbc.C500173200. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. Proc Natl Acad Sci USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz M, Watson NB, Turkington G, Verkoczy LK, Klinman NR, McGregor WG. Mol Cancer Res. 2003;1:836–847. [PubMed] [Google Scholar]
- 15.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 16.Tissier A, Frank EG, McDonald JP, Iwai S, Hanaoka F, Woodgate R. EMBO J. 2000;19:5259–5266. doi: 10.1093/emboj/19.19.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JH, Besaratinia A, Lee DH, Lee CS, Pfeifer GP. Mutat Res. 2006;599:58–65. doi: 10.1016/j.mrfmmm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Bebenek K, Tissier A, Frank EG, McDonald JP, Prasad R, Wilson SH, Woodgate R, Kunkel TA. Science. 2001;291:2156–2159. doi: 10.1126/science.1058386. [DOI] [PubMed] [Google Scholar]
- 19.Vaisman A, Woodgate R. EMBO J. 2001;20:6520–6529. doi: 10.1093/emboj/20.22.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celewicz L, Mayer M, Shetlar MD. Photochem Photobiol. 2005;81:404–418. doi: 10.1562/2004-06-15-ra-201.1. [DOI] [PubMed] [Google Scholar]
- 21.Vaisman A, Takasawa K, Iwai S, Woodgate R. DNA Repair (Amsterdam) 2006;5:210–218. doi: 10.1016/j.dnarep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Lin Q, Clark AB, McCulloch SD, Yuan T, Bronson RT, Kunkel TA, Kucherlapati R. Cancer Res. 2006;66:87–94. doi: 10.1158/0008-5472.CAN-05-1862. [DOI] [PubMed] [Google Scholar]
- 23.Hagendoorn J, Tong R, Fukumura D, Lin Q, Lobo J, Padera TP, Xu L, Kucherlapati R, Jain RK. Cancer Res. 2006;66:3360–3364. doi: 10.1158/0008-5472.CAN-05-2655. [DOI] [PubMed] [Google Scholar]
- 24.Delbos F, De Smet A, Faili A, Aoufouchi S, Weill JC, Reynaud CA. J Exp Med. 2005;201:1191–1196. doi: 10.1084/jem.20050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martomo SA, Yang WW, Wersto RP, Ohkumo T, Kondo Y, Yokoi M, Masutani C, Hanaoka F, Gearhart PJ. Proc Natl Acad Sci USA. 2005;102:8656–8661. doi: 10.1073/pnas.0501852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald JP, Frank EG, Plosky BS, Rogozin IB, Masutani C, Hanaoka F, Woodgate R, Gearhart PJ. J Exp Med. 2003;198:635–643. doi: 10.1084/jem.20030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martomo SA, Yang WW, Vaisman A, Maas A, Yokoi M, Hoeijmakers JH, Hanaoka F, Woodgate R, Gearhart PJ. DNA Repair (Amsterdam) 2006;5:392–398. doi: 10.1016/j.dnarep.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Wang YC, Maher VM, Mitchell DL, McCormick JJ. Mol Cell Biol. 1993;13:4276–4283. doi: 10.1128/mcb.13.7.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peto R, Peto J. J R Stat Assoc, Series A. 1972;135:195–206. [Google Scholar]
- 30.Snedecor GW, Cochran WG. Statistical Methods. 6th Ed. Ames, IA: Iowa State Univ Press; 1976. pp. 100–106. [Google Scholar]
- 31.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: Wiley; 1973. pp. 120–123. [Google Scholar]
- 32.Maher VM, Ouellette LM, Curren RD, McCormick JJ. Nature. 1976;261:593–595. doi: 10.1038/261593a0. [DOI] [PubMed] [Google Scholar]
- 33.McGregor WG, Chen RH, Lukash L, Maher VM, McCormick JJ. Mol Cell Biol. 1991;11:1927–1934. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes M, Foiani M, Sogo JM. Mol Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Garinis GA, Jans J, van der Horst GT. Future Oncol. 2006;2:191–199. doi: 10.2217/14796694.2.2.191. [DOI] [PubMed] [Google Scholar]
- 36.Vrieling H, Van Rooijen ML, Groen NA, Zdzienicka MZ, Simons JW, Lohman PH, van Zeeland AA. Mol Cell Biol. 1989;9:1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen SM, Brylawski BP, Cordeiro-Stone M, Kaufman DG. J Cell Biochem. 2002;85:346–356. doi: 10.1002/jcb.10136. [DOI] [PubMed] [Google Scholar]
- 38.McGregor WG, Wei D, Maher VM, McCormick JJ. Mol Cell Biol. 1999;19:147–154. doi: 10.1128/mcb.19.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell DL, Jen J, Cleaver JE. Photochem Photobiol. 1991;54:741–746. doi: 10.1111/j.1751-1097.1991.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhong X, Garg P, Stith CM, Nick McElhinny SA, Burgers PMJ, Kunkel TA. Nucleic Acids Res. 2006;34:4731–4742. doi: 10.1093/nar/gkl465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loeb LA. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 42.McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC. Mol Cell. 2005;20:783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, Kikuchi K, Masutani C, Hanaoka F, Nozaki K, Hashimoto N, et al. Mol Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Ogi T, Lehmann AR. Nat Cell Biol. 2006;8:640–642. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 45.Opperman T, Murli S, Smith BT, Walker GC. Proc Natl Acad Sci USA. 1999;96:9218–9223. doi: 10.1073/pnas.96.16.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamato TD, Richardson E, Perez ML. Mutat Res. 1995;328:175–181. doi: 10.1016/0027-5107(95)00007-6. [DOI] [PubMed] [Google Scholar]
- 47.Stamato TD, Perez ML. Int J Radiat Biol. 1998;74:739–745. doi: 10.1080/095530098141014. [DOI] [PubMed] [Google Scholar]
- 48.Gening LV, Makarova AV, Malashenko AM, Tarantul VZ. Biochemistry (Moscow) 2006;71:155–159. doi: 10.1134/s0006297906020064. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Devereux TR, Vikis HG, McCulloch SD, Holliday W, Anna C, Wang Y, Bebenek K, Kunkel TA, Guan K, You M. Cancer Res. 2004;64:1924–1931. doi: 10.1158/0008-5472.can-03-3080. [DOI] [PubMed] [Google Scholar]
- 50.Lee GH, Matsushita H. Cancer Sci. 2005;96:256–259. doi: 10.1111/j.1349-7006.2005.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleiss JL. Statistical Methods for Rates and Proportions. New York: Wiley; 1973. [Google Scholar]
- 53.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Boston: McGraw-Hill; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.