Abstract
Apoptosis-inducing factor (AIF), a mitochondrial oxidoreductase, is released into the cytoplasm to induce cell death in response to poly(ADP-ribose) (PAR) polymerase-1 (PARP-1) activation. How PARP-1 activation leads to AIF release is not known. Here we identify PAR polymer as a cell death signal that induces release of AIF. PAR polymer induces mitochondrial AIF release and translocation to the nucleus. PAR glycohydrolase, which degrades PAR polymer, prevents PARP-1-dependent AIF release. Cells with reduced levels of AIF are resistant to PARP-1-dependent cell death and PAR polymer cytotoxicity. These results reveal PAR polymer as an AIF-releasing factor that plays important roles in PARP-1-dependent cell death.
Keywords: excitotoxicity, poly(ADP, ribose) polymerase
Apoptosis-inducing factor (AIF), a flavoprotein of ≈67 kDa that shares homology with the bacterial oxidoreductases (1), may be a cell death effector that is required for poly(ADP-ribose) (PAR) polymerase-1 (PARP-1)-mediated cell death (2–4). Excessive activation of the nuclear enzyme, PARP-1 plays a prominent role in various of models of cellular injury (5, 6). PARP-1-dependent toxicity appears to be caspase-independent (4, 7–9), and PARP-1 activation is required for AIF translocation during cell death initiated by N-methyl-N-nitro-N-nitrosoguanidine (MNNG) (a DNA alkalating agent that potently activates PARP-1 and elicits PARP-1-dependent cytotoxicity) and hydrogen peroxide in fibroblasts (4). In the nervous system, AIF translocation occurs after a variety of toxic insults including NMDA-mediated excitotoxicity, trauma, cerebral ischemia, hypoxia/ischemia, and oxidative stress (4, 10–14). NMDA-mediated AIF translocation is PARP-1 dependent (3). Moreover, NMDA glutamate receptor excitotoxicity appears to require AIF as neutralizing AIF antibodies reduce NMDA excitotoxicity (3) and cortical cultures from Harlequin (Hq) mice, which have reduced levels of AIF due to a proviral insertion (15) are resistant to the toxic effects of NMDA (16) and have smaller cerebral infarcts after middle cerebral artery occlusion (2). How nuclear PARP-1 activation signals to the mitochondria to release AIF is not known. PARP-1-dependent AIF-mediated cell death is heralded by the very early production of PAR polymer (3, 4). Here we explore AIF release in PARP-1-dependent cell death and report that PAR polymer acts as AIF-releasing factor.
Results
PAR Polymer Is an AIF-Releasing Factor.
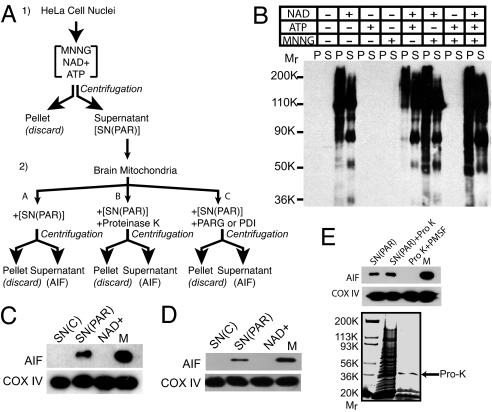
A cell-free based system was developed to determine the nature of the signal induced by activation of nuclear PARP-1 that mediates AIF release (Fig. 1). For these studies, we activated PARP-1 from isolated HeLa cell nuclei and exposed isolated brain mitochondria to the PARP-1-activated HeLa cell nuclei supernatant and monitored AIF release (Fig. 1A). HeLa cell nuclei were used as they are a reliable source of highly purified nuclei of sufficient quantity for downstream assays and analyses. Isolated HeLa cell nuclei were treated with MNNG, ATP, and/or NAD+ to induce PARP-1-activation and formation of PAR polymer formation (Fig. 1B). PAR polymer formation was monitored by Western blot analysis using a selective and specific antibody to PAR (4, 17, 18). PAR polymer is only detected in the presence of NAD+ in both the nuclear pellet and nuclear supernatant and it is slightly enhanced by the addition of MNNG and ATP (Fig. 1B). Isolated mitochondria from mouse brain were exposed to the HeLa cell nuclear supernatant in which PARP-1 was activated followed by different treatments of the supernatant (Fig. 1A). After centrifugation, the supernatant of the mitochondria was monitored for AIF release (Fig. 1C). Cytochrome oxidase subunit IV (COX IV) was used to monitor the integrity of the mitochondria and to confirm that equal amounts of mitochondria were used for each incubation. PARP-1-activated nuclear supernatant [SN(PAR)] induces AIF release from isolated mitochondria (Fig. 1C), whereas control supernatant [SN(C)] fails to induce AIF release. NAD+ alone fails to induce AIF release (Fig. 1C). SN(PAR) from isolated neuronal nuclei also induces AIF release in a manner similar to HeLa cell nuclei (Fig. 1D). To begin to characterize the nature of the AIF-releasing factor, SN(PAR) was treated with proteinase K (Pro K) (20 μg) for 1 h followed by the inactivation of Pro K with PMSF (Fig. 1 A and E). Under these conditions, the majority of proteins are degraded by Pro K as assessed by Coomassie blue staining (Fig. 1E). Surprisingly, Pro K-treated supernatant still retains AIF-releasing activity (Fig. 1E). Because it is possible that Pro K treatment of mitochondria could nonspecifically release AIF, mitochondria were treated under identical conditions with Pro K that was inactivated with PMSF, but lacks SN(PAR). Under these conditions, Pro K alone fails to release AIF from the mitochondria (Fig. 1E). These results suggest that the AIF-releasing activity of SN(PAR) may not be proteinacious.
Fig. 1.
Poly(ADP-ribosyl)ated extranuclear supernatant SN(PAR) induces AIF release from isolated mouse brain mitochondria. (A) Schematic diagram of in vitro PARP-1 activation and preparation of extranuclear supernatant (1). Isolated HeLa cell nuclei were incubated with various combinations of NAD+, ATP, and MNNG to induce DNA damage and PARP-1 activation. After centrifugation, the extranuclear supernatant [SN(PAR)] was separated from the nuclear pellet (2). (A–C) SN(PAR) (A), SN(PAR) + Pro K (B), or SN(PAR) + PARG or PD1 (C) was added to isolated brain mitochondria. After centrifugation, the extramitochondrial supernatant was separated and monitored for AIF release. (B) HeLa cell nuclei incubated with NAD+ alone or in combination with ATP and MNNG can synthesize PAR polymer in both the pellet (P) and supernatant (S). The nuclear supernatant containing poly (ADP-ribosyl)ated proteins after treatment of the nuclei with NAD+, ATP, and MNNG is designated as SN(PAR). SN(C) is the nuclear supernatant after incubation of the nuclei with buffer alone without reagents. (C) SN(PAR) can induce AIF release from isolated mouse brain mitochondria. The mitochondria were incubated for 10 min for all AIF release assays. M stands for the total mitochondria and is the input control (5 μg per lane). Subunit IV of cytochrome oxidase (COX IV) serves as a loading control to ensure the equal amount of mitochondria for each incubation. (D) Cortical neuron SN(PAR) can induce AIF release from isolated mouse brain mitochondria. (E) The AIF releasing activity of SN(PAR) is unaffected by Pro K pretreatment (Upper). SN(PAR) was treated with 20 μg of Pro K for 1 h and PMSF was added to inhibit Pro K activity. Most of the proteins of SN(PAR) are digested under this condition, as revealed by Coomassie blue protein staining (Lower). Arrow indicates Pro K. Pro K itself, which is inhibited by PMSF, has no AIF release activity. These experiments have been replicated in separate experiments at least three times with similar results.
Because PARP-1 activation leads to PAR polymer formation, we wondered whether the PAR polymer or a PAR modified nuclear protein could act as a AIF-releasing factor. To determine whether PAR polymer is the signal, SN(PAR) was treated with phosphodiesterase 1 (PD1) or poly(ADP-ribose) glycohydrolase (PARG), which degrade PAR polymers (Figs. 1A and 2A) (19, 20). Under conditions in which PD1 and PARG markedly reduce PAR polymer from SN(PAR) (Fig. 2A), AIF is not released from isolated brain mitochondria (Fig. 2B). These results suggest that PAR polymer or a poly(ADP-ribose) modified nuclear protein is the AIF-releasing factor induced by PARP-1 activation. The AIF release by the PAR moiety of SN(PAR) raised the possibility that PAR polymer itself could function as the AIF-releasing factor. PAR polymer was synthesized and purified after in vitro automodification of PARP-1 and has a mean length of 40 ADP-ribose residues as determined by HPLC methods and gel electrophoresis (21). The range of size of PAR in this mix is 6-mer through 100-mer ADP-ribose units (21, 22). Purified PAR polymer induces AIF release in a manner similar to SN(PAR) (Fig. 2C). In an identical manner to the AIF-releasing activity of SN(PAR), Pro K treatment fails to prevent PAR polymer-induced AIF release (Fig. 2D). Under conditions in which PARG and PD1 degrade PAR polymer (data not shown) (23), it is not capable of inducing AIF release (Fig. 2E). Thus, free PAR polymer appears to be an AIF-releasing factor.
Fig. 2.
PAR polymer induces AIF release from isolated mouse brain mitochondria. (A) Degradation of PAR moiety of SN(PAR) with PD1 (0.01 unit) or recombinant PARG (1 unit). Removal of poly(ADP-ribosyl)ation in SN(PAR) was verified using an anti-PAR antibody. (B) Treatment of SN(PAR) with PD1 (0.01 unit) or recombinant PARG (1 unit) for 1 h abolishes AIF releasing activity. (C) Purified PAR polymer (PAR, 100 nM) induces AIF release in isolated mouse brain mitochondria. AIF is not detected in the supernatant of the mitochondria incubated with buffer alone (B). (D) The AIF releasing activity of PAR polymer is unaffected by Pro K (20 μg, 1 h) pretreatment. (E) Purified PAR polymer (PAR, 100 nM) pretreated with PD1 (0.01 unit) or recombinant PARG (1 unit) for 1 h loses AIF releasing activity. (F) Different PAR polymer fractions with average polymer size (16-, 30-, and 60-mer) were delivered into the cortical neurons via the BioPorter delivery reagent at a final concentration of 80 nM and AIF release was monitored. B and M stand for buffer and total mitochondria protein (5 μg), respectively. (G) Dose-dependent AIF release by PAR polymer of 60 ADP-ribose units. These experiments have been replicated in separate experiments at least three times with similar results.
To determine the nature of the PAR polymer that mediates AIF release, PAR polymer of different size and complexity ranging in length from 16 to >60 ADP-ribose residues were evaluated (Fig. 2F) (22). An 80 nM concentration of 16 ADP-ribose residues causes a small amount of AIF release, whereas 80 nM of polymers of 30 ADP-ribose residues induce modest AIF release and 80 nM of polymers greater then 60 ADP-ribose units or the mixed polymer fraction induce robust AIF release (Fig. 2F). To ascertain whether increasing concentrations of complex polymers greater than 60 ADP-ribose units also lead to more AIF release, we monitored AIF release from isolated mouse brain mitochondria as assessed by subcellular fractionation (Fig. 2G). PAR polymer (20 nM) induces mild AIF release and AIF release increases with increasing concentration of PAR polymer (Fig. 2G). These concentrations, length and complexity of PAR polymers are within the range of polymer concentrations and size found in intact cells during NMDA excitotoxicity (3, 4, 17, 23), and PARP-1-dependent (MNNG-induced) HeLa cell toxicity (data not shown). Thus, it is likely that complex and high molecular weight PAR polymer acts as the nuclear signal to induce mitochondrial release of AIF.
Nuclear and Cytosolic PAR Polymer.
The identification of PAR polymer as a nuclear signal that induces mitochondrial AIF release suggests that PAR polymer translocates from the nucleus to the mitochondria to induce AIF release. To determine whether PAR polymer is present in the cytoplasm of intact cells, we evaluated whether NMDA-induced excitotoxicity of primary cortical neurons, a pathophysiologically relevant form of PARP-1-dependent cell death (3), leads to cytosolic PAR polymer formation. PAR polymer formation and localization were monitored via immunohistochemical detection using confocal microscopy and subcellular fractionation followed by Western blot analysis for PAR polymer (Fig. 3). Fifteen minutes after a 5-min application of NMDA (500 μM), PAR polymer is primarily observed in the nucleus of cortical neurons (Fig. 3 A and C), but 30 and 60 min after NMDA receptor stimulation, it is localized to cytoplasm as assessed by confocal analysis (Fig. 3A). High power examination of individual cells clearly shows that PAR polymer is present as a thin cytoplasmic rim around the nucleus of cortical neurons (Fig. 3B and Fig. 7, which is published as supporting information on the PNAS web site). Quantitation of the number of cells possessing cytoplasmic PAR polymer indicates that greater than 70% of the cortical neurons following NMDA receptor stimulation have PAR polymer in the cytoplasm, which is consistent with the number of neurons that typically show nuclear condensation following NMDA receptor stimulation (3, 4) (data not shown). Subcellular fractionation of cortical neurons following NMDA receptor stimulation into cytosolic, mitochondrial and nuclear fractions was performed to confirm the confocal analysis (Fig. 3C). Immunoreactivity for histones, GAPDH, and MnSOD were used to monitor the integrity of the nuclear, cytosolic, and mitochondrial subcellular fractions, respectively (Fig. 3C). PAR polymer formation is mainly present in the nuclear fraction as early as 15 min after NMDA receptor stimulation, but it is also clearly present in the cytosolic fraction, as well as the mitochondrial fraction, 30 min after NMDA application (Fig. 3C). To determine whether PAR polymer is localized to the mitochondria, colocalization studies were conducted with PAR polymer and cytochrome oxidase 1 (COX1), an integral mitochondrial protein. Sixty minutes after NMDA (500 μM) administration, PAR polymer is colocalized, in part, with COX1 at the mitochondria (Fig. 3D). Although the exact physiological functions of PAR polymer in other compartments of the cytosol remain unknown, our data demonstrate that PAR polymer generated by NMDA receptor stimulation is in the nucleus and cytosol and that it colocalizes with the mitochondria, where it may act to induce AIF release.
Fig. 3.
Cytosolic and mitochondrial PAR polymer after PARP-1 activation. (A) Representative confocal images of cortical neurons 15, 30, and 60 min after NMDA receptor stimulation. DIC images are provided to illustrate neurons. Immunocytochemical staining indicates that PAR polymer (red) not only exists in the nucleus (blue), but also in the cytoplasm in the NMDA (500 μM) treated neurons. (B) Representative images of high-power examination of individual neurons after 60 min of NMDA treatment, showing that PAR polymer is present as a thin cytoplasmic rim around the nucleus of cortical neurons. Nuclear PAR localization appears as pink staining and cytoplasmic localization appears as red staining. (Scale bar, 10 μm.) (C) Subcellular fractionations of cortical neurons indicate that PAR polymer can be found in nuclear (N), cytoplasmic (C), and mitochondrial (M) fractions. Western blotting with antibodies against histones, GAPDH, and MnSOD confirms the integrity of nuclear, cytosolic, and mitochondrial fractions, respectively. (D) Mitochondrial location of PAR polymer. Cortical neurons were immunostained with the mitochondrial protein, cytochrome oxidase 1 (COX-1, green), and PAR polymer (red); nuclei were counterstained with DAPI (blue, 300 nM in PBS) in NMDA (500 μM for 5 min)-treated neurons. PAR polymer colocalizes to mitochondria (merged image, Lower) 60 min after NMDA-treatment, whereas no PAR formation is seen in CSS-treated neurons (Upper, control). Yellow indicates overlap between COX-1 and PAR staining. A single neuron under higher magnification (magnified) is shown (Right). These experiments have been replicated in separate experiments at least three times with similar results. (Scale bar, 10 μm.)
PAR Polymer Induces AIF Release.
To investigate whether PAR polymer could induce AIF release in situ from cortical neurons, PAR polymer was applied to cortical neurons via the BioPorter delivery system (24). PAR polymer is effectively delivered into cortical neurons via the BioPorter reagent as revealed using PAR polymer antibody and confocal imaging (data not shown) (23). PAR polymer induces AIF translocation and nuclear condensation as monitored via confocal image analysis (Fig. 4A and B). Under conditions in which PARG and PD1 degrade PAR polymer (23) PAR polymer administration via BioPorter delivery to cortical neurons fails to induce AIF translocation and nuclear condensation (Fig. 4 A and B). Because PAR polymer is a highly negatively charged molecule, the same concentration of poly (adenine) [poly(A)], also negatively charged, was applied via BioPorter to cortical neurons. Poly(A) fails to induce AIF release, indicating that negatively charged polymers are not sufficient to induce AIF release (Fig. 4B). To confirm the confocal image analysis, subcellular fractionation of cortical neurons was performed after PAR polymer delivery followed by Western blot analysis for AIF, histone, and MnSOD. PAR polymer induces AIF nuclear translocation in cortical neurons, whereas PAR polymer pretreated with PARG or PD1 fails to induce AIF translocation (Fig. 4C). Poly(A) also fails to induce AIF release as monitored via subcellular fractionation (Fig. 4C). To ascertain whether there is a reduction in mitochondrial AIF after PAR polymer delivery, the neuronal cultures were subfractionated into nuclear and mitochondrial fractions 6 h after PAR delivery to monitor the change in AIF levels. Accompanying the PAR polymer-induced translocation of mitochondrial AIF to the nucleus is a reduction in mitochondrial AIF (Fig. 4D), thus loss of AIF in the mitochondrial fraction is attendant on nuclear translocation of AIF by PAR in cortical neurons. As described (4, 17, 25), cortical neurons from PARP-1-knockout mice are dramatically resistant to NMDA excitotoxicity as NMDA (500 μM, 5 min) causes significant neuronal death in wild-type neurons, whereas PARP-1 knockout neurons are completely resistant to the toxic effects of NMDA. Under these conditions AIF is not released from mitochondria (3, 4). If, however, PAR polymer induces AIF release downstream of PARP-1, then delivery of PAR polymer into PARP-1 KO neurons should bypass PARP-1 inactivation and restore the same pattern of AIF translocation and nuclear condensation as observed in wild-type neurons. To test this idea, PAR polymer was administered to PARP-1 KO cortical neurons (Fig. 4E). PAR polymer induces AIF nuclear translocation in PARP-1 KO cortical neurons, whereas PAR polymer pretreated with PARG or PD1 fails to induce AIF translocation (Fig. 4 E and F). Poly(A) also fails to induce AIF release in PARP-1 KO cortical neurons as monitored via subcellular fractionation (Fig. 4F). These results, taken together, indicate that cytosolic PAR polymer is sufficient to induce AIF release from the mitochondria and induce its translocation to the nucleus.
Fig. 4.
Delivery of purified PAR polymer into neurons induces AIF translocation and nuclear condensation. (A) Representative confocal images of AIF translocation and nuclear condensation after BioPorter mediated delivery of purified PAR polymer into wild-type (WT) cortical cultures with and without pretreatment with PARG. AIF (red) is seen in well defined mitochondria like structures in the neuronal cell body as well as long axonal processes and no AIF is seen in the nucleus (blue) in control or PAR + PARG-treated cultures, whereas a clear nuclear translocation and shrinkage of AIF (Pink) is seen in PAR treated cultures. (Scale bar, 20 μm.) (B) Quantitative analysis of AIF translocation and nuclear condensation in the neurons treated with PAR polymer with and without pretreatment with PARG or PD1. BioPorter mediated delivery of poly(A) serves as a control. ∗, P < 0.05, Student's t test. Data represent the mean ± SD; n = 3. (C) Western blots of subcellular fractions of WT cultures, showing nuclear AIF translocation after PAR delivery. Synthetic polymer, poly(A), or enzymatic digestion of PAR with PARG or PD1 did not induce any nuclear AIF translocation. Integrity of mitochondrial fraction (M) and nuclear fraction is shown with antibodies against MnSOD or histone, respectively. (D) Nuclear translocation of AIF accompanies decrease of AIF in mitochondrial fraction. PAR polymer was delivered into cortical neurons using BioPorter for 6 h and subjected to subcellular fractionation into nuclear and mitochondrial fractions. MnSOD and histone were probed as mitochondrial and nuclear markers, respectively. PAR polymer induces the nuclear translocation of AIF, concurrent with loss of AIF in the mitochondrial fraction. (E) Representative confocal images of AIF translocation and nuclear condensation after BioPorter mediated delivery of purified PAR polymer into PARP-1 knockout (KO) cultures with and without pretreatment with PARG. (Scale bar, 20 μm.) (F) Western blots of subcellular fractions of PARP-1 KO cultures, showing nuclear AIF translocation after PAR delivery. Synthetic polymer poly(A) or enzymatic digestion of PAR with PARG or PD1 did not induce any nuclear AIF translocation. These experiments have been replicated in separate experiments at least three times with similar results.
We next evaluated whether interfering with PAR polymer formation induced by PARP-1 activation prevents and/or reduces AIF translocation in cortical neurons by monitoring AIF translocation following NMDA receptor stimulation with confocal microscopy and subcellular fractionation. Adenoviral-mediated overexpression of wild-type cytosolic PARG prevents NMDA-induced AIF translocation as assessed by confocal microscopy whereas overexpression of the catalytically inactive mutant PARG fails to prevent AIF translocation as assessed by confocal microscopy (Fig. 5A); and via subcellular fractionation using immunoreactivity for histones and MnSOD to monitor the purity of the nuclear and mitochondrial fractions, respectively (Fig. 5B). To control for nonspecific effects of adenoviral-mediated gene expression, adenoviral-mediated overexpression of GFP was evaluated and it has no effect as NMDA-induced AIF translocation (Fig. 5). Thus, reducing PAR polymer levels decreases AIF translocation after NMDA excitotoxicity.
Fig. 5.
NMDA-induced AIF mitochondrial nuclear translocation is mediated by PAR polymer. (A) Confocal images of neurons of AIF translocation in cortical neurons that were treated with NMDA (500 μM) with or without PARG overexpression. Cortical neurons were infected with Av PARG WT, Av PARG Mut virus, or Av GFP virus as a control or treated with PBS, followed by NMDA-stimulation (500 μM). In control cultures (CSS), the neurons were incubated in CSS alone for 5 min. Mitochondria-like AIF staining is seen in CSS treated cultures (red), whereas in NMDA-treated cultures (PBS), a robust nuclear translocation of AIF is seen (pink). PARG overexpression (Av PARG WT) protects the neurons against NMDA-induced AIF translocation, whereas overexpression of catalytically inactive PARG (Av PARG Mut) fails to prevent AIF translocation. In control adenoviral experiments, the neurons show a robust nuclear AIF translocation (Av GFP) similar to NMDA-treated (PBS) cultures. (B) Overexpression of cytosolic, WT PARG reduces AIF translocation induced by NMDA treatment in cortical neurons. Neurons were infected with adenoviral constructs as in A. Six hours after NMDA treatment, neurons were harvested for subcellular fractionation and monitoring of AIF translocation. MnSOD and histone serve as mitochondria and nuclear fraction markers, respectively. These experiments have been replicated in separate experiments at least three times with similar results.
To ascertain whether AIF mediates PAR polymer-induced cell death, we used cortical cultures from Hq mutant mice, which contain a proviral insertion in the AIF gene resulting in an 80% of reduction in AIF expression (15). First, we confirmed that NMDA excitotoxicity is mediated by AIF (2, 3). Hq cortical cultures are relatively resistant to NMDA excitotoxicity compared with wild-type littermate cortical cultures (Fig. 6A and B) confirming prior results (2, 3). NMDA induces an equivalent amount of PAR polymer in both wild-type and Hq mice (Fig. 6C). Because the reduction in AIF in Hq mice is due to a proviral insertion (15), it is conceivable that reduction in NMDA excitotoxicity in Hq mice may be due to some other differences unrelated to AIF in the mice. To control for this possibility, we used adenoviral-mediated overexpression of GFP-tagged AIF to replace the reduced AIF. Previously, we and others have shown that GFP-tagged AIF translocates to the nucleus after glutamate excitotoxicity and is fully competent as a death effector (16, 26). Adenoviral-mediated overexpression of AIF leads to increased levels of AIF in Hq cortical cultures (Fig. 6D). After restoration of AIF, Hq cortical cultures are rendered susceptible to NMDA excitotoxicity, whereas adenoviral GFP infected Hq cortical cultures are still resistant to the toxic effects of NMDA (Fig. 6E). Taken together, these results suggest that reduction in cell death observed in Hq mice after NMDA excitotoxicity is due to the reduction in AIF levels. To determine whether AIF plays a role in PAR polymer-mediated toxicity, we evaluated whether Hq cortical cultures are resistant to the toxic effects of BioPorter-mediated delivery of PAR polymer (Fig. 6 F and G). Hq cortical neurons are resistant to the toxic effects of PAR polymer compared with wild-type cortical cultures. These results suggest that NMDA excitotoxicity and PAR polymer-induced cell death is mediated, in part, via AIF.
Fig. 6.
AIF mediates NMDA and PAR polymer-induced cytotoxicity. (A) Cortical neurons from Hq mice and their WT littermates were subjected to NMDA-excitotoxicity (100 μM, 5 min) on day in vitro (DIV) 14. After 18–24 h of NMDA application, the neurons were stained with PI (red) and Hoechst (blue). PI-positive cells are considered dead. (B) Quantification of the NMDA-induced cell death in Hq neurons. Hq neurons that have reduced expressions of AIF are significantly protected against NMDA-toxicity than their WT littermates. Control cultures were treated with CSS alone. Data are the mean ± SEM; n = 6; ∗, P < 0.05 vs. control. (C) PAR polymer in neurons from Hq mice and their WT littermates that were subjected to NMDA-stimulation on DIV 14. Samples were harvested 1 h after NMDA-stimulation and immunoblotted for PAR polymer using anti-PAR antibody. Hq mice that are resistant to NMDA-toxicity have the same PAR polymer levels as compared with their WT littermates. Equal protein loading is shown with the blots for β-actin. These experiments have been replicated in separate experiments at least three times with similar results. (D) Cortical neurons from Hq mouse embryos or their wild type litter mates were infected with adenoviral AIF-GFP (Av-AIF) or GFP-adenoviral control construct (Av-GFP). Forty-eight hours later, the cells were harvested, subjected to SDS/PAGE, and probed for AIF using an AIF-specific antibody. A GFP-AIF fusion protein band is detected in Hq-neurons that were treated with Av AIF-GFP adenovirus; whereas reduced levels of endogenous AIF are seen in PBS or Adeno-GFP-infected cultures. (E) Compensation of reduced AIF expression in Hq-neurons by AIF-expressing adenovirus makes Hq neurons equally susceptible to NMDA as their WT littermate neurons. Neurons (12 DIV) were infected with Av AIF-GFP or GFP-adenoviral control construct (Adeno-GFP). Control cultures were treated with an equal volume of PBS. Forty-eight hours later, the neurons were treated with NMDA (500 μM for 5 min) and cells were stained with Hoechst and PI to assess cell death, 18–24 h after NMDA administration. Data are the mean ± SEM; n = 6; ∗, P < 0.05. (F) Hq neurons are protected against PAR polymer-induced cell death as compared with their WT littermates. Representative pictures of propidium iodide (red), Hoechst (blue)-stained Hq neurons that were treated with purified PAR polymer delivered via the BioPorter reagent. The PI-positive cells were considered dead. (G) Quantification of the data shown in F. Hq neurons are significantly protected against PAR-polymer-induced cell death as compared with their WT littermates. Data are the mean ± SEM; n = 6; ∗, P < 0.05 vs. control.
Discussion
The major finding of this paper is that PAR polymer is an AIF-releasing factor. Initially experiments were designed to identify and purify the AIF-releasing factor induced by PARP-1 activation (see Fig. 1A). However, through the course of our experiments it became clear that the AIF-releasing factor was not proteinaceous, and that PAR polymer, the major product of PARP-1 activation, was the AIF-releasing factor. Evidence in support that PAR polymer is the AIF-releasing factor include the observations that PARP-1-activated nuclear supernatant induces AIF release from isolated mitochondria and that the AIF-releasing activity is abolished by treatment of PARP-1-activated nuclear supernatant with either PD1 or PARG, which degrade PAR polymer. Moreover, purified PAR polymer induces AIF release from isolated mitochondria and causes the translocation of AIF from the nucleus to the mitochondria in intact cells. In addition, interfering with PAR polymer signaling through PARG overexpression reduces PARP-1-dependent AIF translocation after NMDA excitotoxicity.
Our results suggest that excessive activation of PARP-1 leads to an intrinsic cell death program, which we designate as parthanatos to distinguish from necrosis and apoptosis. PAR polymer appears to be a pro-death signaling molecule that acts as a nuclear/mitochondrial signal to release AIF from the mitochondria in PARP-1 dependent cell death. During PARP-1-dependent cell death, AIF release requires PARP-1 activation as PARP inhibitors or the absence of PARP-1 blocks AIF release (3, 5). When PARP-1 overactivation occurs, PAR polymer is synthesized in the nucleus and released into the cytoplasm where it leads to AIF release, which then translocates to the nucleus, causing nuclear condensation and cell death. Consistent with the notion that AIF is required for PARP-1-dependent cell death is the observation that neurons from Hq mice with reduced levels of AIF are resistant to the toxic effects of PAR polymer and NMDA excitotoxicity. The mechanism by which PAR polymer causes AIF release is not known. However due to its highly charged nature, it could conceivably depolarize mitochondria leading to permeability transition and subsequent AIF release. Alternatively, PAR polymer could bind to PAR polymer binding proteins (27, 28) at the mitochondria, which then triggers AIF release from the mitochondria. Elucidating these mechanisms and interfering with this bidirectional communication may offer therapeutic approaches to treat cellular injury.
Methods
Primary Cortical Cultures and Cytotoxicity.
Primary cortical neuron cultures were prepared from gestational day 14–15 fetal mice, and neurons (14 days in vitro) were exposed to NMDA or PAR polymer and viability was determined by as described (23, 29).
Immunocytochemistry and Confocal Microscopy.
Treated or untreated cortical neurons were stained with an antibody against rabbit polyclonal AIF (4) or rabbit monoclonal AIF antibody (Epitomics, Burlingame, CA) or cytochrome oxidase 1 (COX-1) (Invitrogen, Carlsbad, CA) or anti-PAR polymer antibody (18) and imaged via confocal microscopy using a LSM 510 Meta microscope (Zeiss, Jena, Germany) as described (3). For each sample, at least 10 different regions were scanned, and >200 cells were examined for each data point in at least three separate and independent experiments by two separate examiners.
Subcellular Fractionation.
Fractionation of cortical neurons was based on the method of Yang et al. (30) with modifications as described (4).
PAR Polymer Synthesis and Purification.
Purification and synthesis of PAR polymer were performed as described (18, 22)
In Vitro PARP-1 Activation.
HeLa cells or cortical neuron nuclei were isolated as described (4). The nuclei were incubated to activate PARP-1 with combinations of NAD+ (1 mM), ATP (1 mM), and MNNG (0.5 mM) for 15 min at 37°C in 300 mM sucrose, 10 mM Hepes buffer (pH 7.4), and the nuclear pellet and supernatant were separated by centrifugation at 720 × g for 5 min. The nuclear supernatant, SN(PAR), was diluted to 1 mg/ml and used for the AIF release assay.
AIF Release Assay.
Mitochondria were prepared from mouse brain, following standard differential centrifugation procedures at 4°C. In brief, the mouse brain was removed after decapitation and dounce homogenized in ice-cold homogenation buffer containing 300 mM sucrose, 0.1 mM EDTA in 10 mM Hepes, pH 7.4. The unbroken cells and nuclei were spun down by centrifugation at 600 × g for 5 min. The supernatant was filtered through 4 layers of gauze and pelleted again at the same centrifugal condition. The supernatant was further centrifuged at 3,300 × g for 10 min to collect heavy mitochondrial fraction. The mitochondrial pellet was washed with homogenation buffer once and resuspended to 4 mg/ml in homogenation buffer. Homogenation buffer (50 μl) of mitochondria were incubated with 25 μl of SN(PAR) or purified PAR polymer at indicated concentrations and the buffer was added to bring the reaction volume to 100 μl. The reaction mixtures were incubated at room temperature and centrifuged at 12,000 × g for 10 min at indicated time points.
Western Blotting.
Cell lysates or subcellular fractions were size-separated through denaturing polyacrylamide gel electrophoresis (SDS/PAGE) and Western blots were performed as described (3, 4). All primary antibodies are previously characterized antibodies (anti-histone antibody, US Biological); rabbit polyclonal anti-MnSOD and anti-AIF antibodies (4); mouse monoclonal AIF antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-PAR polyclonal antibody (18). All three AIF antibodies used in these studies recognize a single band on Western blot analysis and there is markedly reduced immunoreactivity in Hq mice (data not shown).
BioPorter Protein Delivery System.
Antibodies, PAR polymer or cleaved PAR polymer either with PD1 (Sigma, St. Louis, MO) or recombinant PARG were diluted to desired concentration with PBS and applied to neurons using the BioPorter reagent (Gene Therapy Systems, San Diego, CA) (24) as described (3, 4).
Transduction of Cultured Neurons with Recombinant Adenovirus.
The exon-1-deleted wild-type PARG gene (Av PARG WT) and exon-1-deleted mutant PARG gene (Av PARG Mut) were used as described (23). The adenoviral GFP-tagged AIF was a gift from Ruth Slack (16, 26). A GFP adenovirus was used as a control.
Mouse Strains.
All procedures on mice were preapproved by the Johns Hopkins University Animal Care and Use Committee. PARP-1 knockouts and Hq mice have been described. PARP-1 knockouts are maintained on an outbred strain of 129 SvEv and 129 SvEv mice were used as controls (17). Hq/+ females and Hq/Y males were bred and aged matched littermate controls were used (15).
Statistical Analysis.
For statistical one-way analysis of variance (ANOVA) was applied followed by Turkey multiple comparison test. Data are shown as mean ± SD or SEM; P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Ruth Slack (University of Ottawa, Ontario, Canada) for the Adeno-GFP-AIF virus. This work was supported by National Institutes of Health Grant NS39148 and the American Heart Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 2.Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellechia M, Blomgren K, Plesnila N. J Neurosci. 2005;25:10262–72. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. J Neurosci. 2004;24:10963–73. doi: 10.1523/JNEUROSCI.3461-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 5.Yu SW, Wang H, Dawson TM, Dawson VL. Neurobiol Dis. 2003;14:303–317. doi: 10.1016/j.nbd.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Virag L, Szabo C. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 7.Kang YH, Yi MJ, Kim MJ, Park MT, Bae S, Kang CM, Cho CK, Park IC, Park MJ, Rhee CH, et al. Cancer Res. 2004;64:8960–8967. doi: 10.1158/0008-5472.CAN-04-1830. [DOI] [PubMed] [Google Scholar]
- 8.Liu TJ, Lin SY, Chau YP. Toxicol Appl Pharmacol. 2002;182:116–125. doi: 10.1006/taap.2002.9438. [DOI] [PubMed] [Google Scholar]
- 9.Wesierska-Gadek J, Gueorguieva M, Schloffer D, Uhl M, Wojciechowski J. J Cell Biochem. 2003;89:1222–1234. doi: 10.1002/jcb.10586. [DOI] [PubMed] [Google Scholar]
- 10.Fonfria E, Dare E, Benelli M, Sunol C, Ceccatelli S. Eur J Neurosci. 2002;16:2013–2016. doi: 10.1046/j.1460-9568.2002.02269.x. [DOI] [PubMed] [Google Scholar]
- 11.Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J. J Cereb Blood Flow Metab. 2003;23:1137–1150. doi: 10.1097/01.WCB.0000087090.01171.E7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Chen J, Graham SH, Du L, Kochanek PM, Draviam R, Guo F, Nathaniel PD, Szabo C, Watkins SC, Clark RS. J Neurochem. 2002;82:181–191. doi: 10.1046/j.1471-4159.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G, Hagberg H, Blomgren K. J Neurochem. 2003;86:306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer I, Planas AM. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 15.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 16.Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC, Park DS, Bennett SA, Slack RS. J Neurosci. 2005;25:1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandir AS, Poitras MF, Berliner AR, Herring WJ, Guastella DB, Feldman A, Poirier GG, Wang ZQ, Dawson TM, Dawson VL. J Neurosci. 2000;20:8005–8011. doi: 10.1523/JNEUROSCI.20-21-08005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Affar EB, Duriez PJ, Shah RG, Winstall E, Germain M, Boucher C, Bourassa S, Kirkland JB, Poirier GG. Biochim Biophys Acta. 1999;1428:137–146. doi: 10.1016/s0304-4165(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 19.Winstall E, Affar EB, Shah R, Bourassa S, Scovassi AI, Poirier GG. Exp Cell Res. 1999;246:395–398. doi: 10.1006/excr.1998.4321. [DOI] [PubMed] [Google Scholar]
- 20.Miwa M, Tanaka M, Matsushima T, Sugimura T. J Biol Chem. 1974;249:3475–3482. [PubMed] [Google Scholar]
- 21.Alvarez-Gonzalez R, Jacobson MK. Biochemistry. 1987;26:3218–3224. doi: 10.1021/bi00385a042. [DOI] [PubMed] [Google Scholar]
- 22.Kiehlbauch CC, Aboul-Ela N, Jacobson EL, Ringer DP, Jacobson MK. Anal Biochem. 1993;208:26–34. doi: 10.1006/abio.1993.1004. [DOI] [PubMed] [Google Scholar]
- 23.Andrabi SA, Kim NS, Yu S-W, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, et al. Proc Natl Acad Sci USA. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelphati O, Wang Y, Kitada S, Reed JC, Felgner PL, Corbeil J. J Biol Chem. 2001;276:35103–10. doi: 10.1074/jbc.M104920200. [DOI] [PubMed] [Google Scholar]
- 25.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 26.Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. J Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagne JP, Hendzel MJ, Droit A, Poirier GG. Curr Opin Cell Biol. 2006;18:145–151. doi: 10.1016/j.ceb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Gagne JP, Hunter JM, Labrecque B, Chabot B, Poirier GG. Biochem J. 2003;371:331–340. doi: 10.1042/BJ20021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Zulueta M, Ensz LM, Mukhina G, Lebovitz RM, Zwacka RM, Engelhardt JF, Oberley LW, Dawson VL, Dawson TM. J Neurosci. 1998;18:2040–2055. doi: 10.1523/JNEUROSCI.18-06-02040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.