Abstract
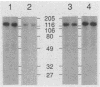
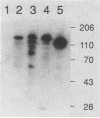
Binding sites for insecticidal toxins of Bacillus thuringiensis are located in the brush border membranes of insect midguts. Two approaches were used to investigate the interactions of B. thuringiensis subsp. kurstaki HD-73 CryIA(c) toxin with brush border membrane vesicles from sensitive and naturally resistant insects: 125I-toxin-vesicle binding assays and protein blots probed with 125I-CryIA(c) toxin. In bioassays, Manduca sexta and Heliothis virescens larvae were highly sensitive, Helicoverpa zea larvae were moderately sensitive, and Spodoptera frugiperda larvae were resistant to CryIA(c) toxin. Studies of binding of 125I-CryIA(c) toxin to brush border membrane vesicles from the larval midguts revealed that all insects tested had high-affinity, saturable binding sites. Significantly, S. frugiperda larvae bind but are not killed by CryIA(c) toxin. Labeled CryIA(c) toxin incubated with protein blots identifies a major binding molecule of 120 kDa for M. sexta and 148 kDa for S. frugiperda. H. virescens and H. zea are more complex, containing 155-, 120-, 103-, 90-, and 63-kDa proteins as putative toxin-binding molecules. H. virescens also contains a minor toxin-binding protein of 81 kDa. These experiments provide information that can be applied toward a more detailed characterization of B. thuringiensis toxin-binding proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haider M. Z., Ellar D. J. Analysis of the molecular basis of insecticidal specificity of Bacillus thuringiensis crystal delta-endotoxin. Biochem J. 1987 Nov 15;248(1):197–201. doi: 10.1042/bj2480197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C., Vanderbruggen H., Höfte H., Van Rie J., Jansens S., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. H., Ellar D. J. Characterization and partial purification of a plasma membrane receptor for Bacillus thuringiensis var. kurstaki lepidopteran-specific delta-endotoxin. J Cell Sci. 1986 Jul;83:89–101. doi: 10.1242/jcs.83.1.89. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leslie F. M. Methods used for the study of opioid receptors. Pharmacol Rev. 1987 Sep;39(3):197–249. [PubMed] [Google Scholar]
- MacIntosh S. C., Stone T. B., Sims S. R., Hunst P. L., Greenplate J. T., Marrone P. G., Perlak F. J., Fischhoff D. A., Fuchs R. L. Specificity and efficacy of purified Bacillus thuringiensis proteins against agronomically important insects. J Invertebr Pathol. 1990 Sep;56(2):258–266. doi: 10.1016/0022-2011(90)90109-j. [DOI] [PubMed] [Google Scholar]
- Stieger B., Murer H. Heterogeneity of brush-border-membrane vesicles from rat small intestine prepared by a precipitation method using Mg/EGTA. Eur J Biochem. 1983 Sep 1;135(1):95–101. doi: 10.1111/j.1432-1033.1983.tb07622.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie J., Jansens S., Höfte H., Degheele D., Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990 May;56(5):1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie J., Jansens S., Höfte H., Degheele D., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur J Biochem. 1989 Dec 8;186(1-2):239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- Van Rie J., McGaughey W. H., Johnson D. E., Barnett B. D., Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990 Jan 5;247(4938):72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- Wolfersberger M. G. The toxicity of two Bacillus thuringiensis delta-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membranes for the toxins. Experientia. 1990 May 15;46(5):475–477. doi: 10.1007/BF01954236. [DOI] [PubMed] [Google Scholar]