Abstract
The nematophagous fungus Arthrobotrys oligospora produced extracellular proteases when grown in a liquid culture, as revealed by measuring the hydrolysis of the chromogenic substrate Azocoll. The extracellular protease activity was inhibited by phenylmethylsulfonyl fluoride (PMSF) and other serine protease inhibitors and partly inhibited by the aspartate protease inhibitor pepstatin and by a cysteine protease inhibitor [l-trans-epoxysuccinyl-leucylamide-(4-guanidino)-butane, or E-64]. Substrate gel electrophoresis showed that the fungus produced several different proteases, including multiple serine proteases. The function of proteases in the infection of nematodes was examined by treating the fungus with various protease inhibitors. None of the inhibitors tested affected the adhesion of nematodes to the traps, but incubating trap-bearing mycelium with a serine protease inhibitor, PMSF, antipain, or chymostatin, or the metalloprotease inhibitor phenanthroline significantly decreased the immobilization of nematodes captured by the fungus. Inhibitors of cysteine or aspartic proteases did not affect the immobilization of captured nematodes. The effects of PMSF on the immobilization of nematodes were probably due to serine proteases produced by the fungus, since the effects were observed when unbound inhibitor was washed away from the fungus before the nematodes were added to the system. No effects were observed when the nematodes only were pretreated with PMSF.
Full text
PDF

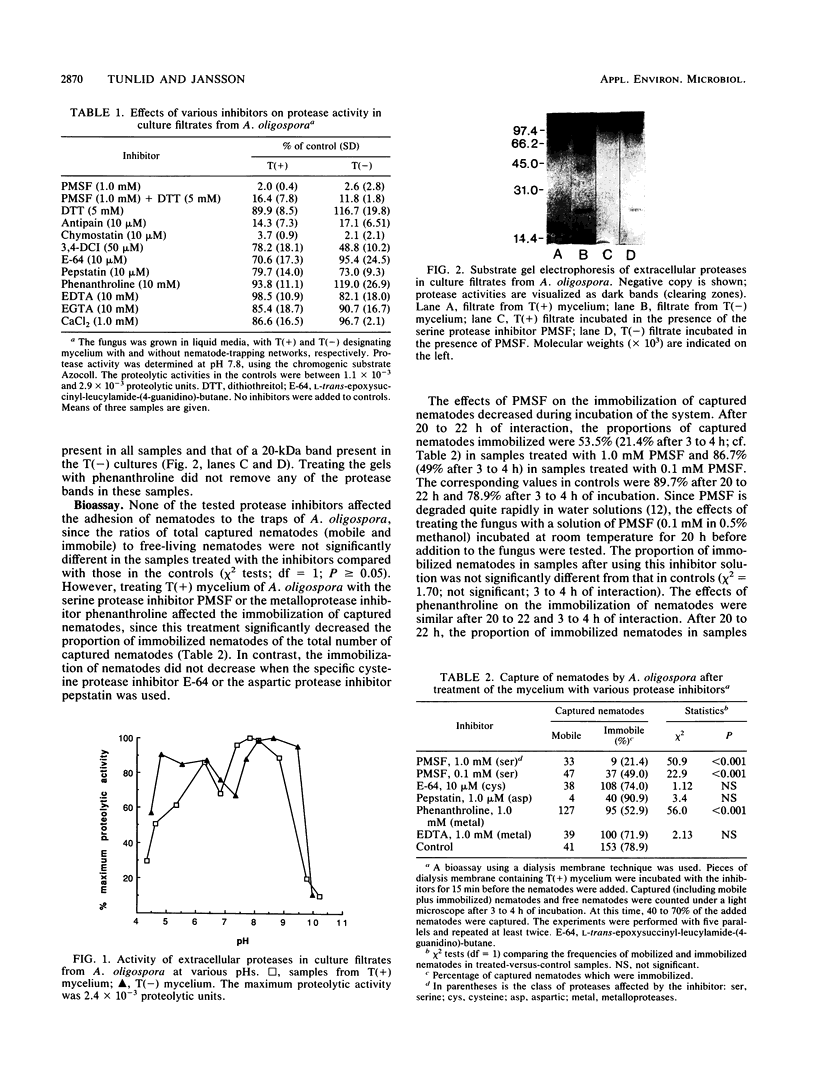


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo R., Alderete J. F. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect Immun. 1989 Oct;57(10):2991–2997. doi: 10.1128/iai.57.10.2991-2997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidochka M. J., Khachatourians G. G. Purification and Properties of an Extracellular Protease Produced by the Entomopathogenic Fungus Beauveria bassiana. Appl Environ Microbiol. 1987 Jul;53(7):1679–1684. doi: 10.1128/aem.53.7.1679-1684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chavira R., Jr, Burnett T. J., Hageman J. H. Assaying proteinases with azocoll. Anal Biochem. 1984 Feb;136(2):446–450. doi: 10.1016/0003-2697(84)90242-2. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Kusch M., Edgar R. S. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol. 1981 Jul;90(1):7–17. doi: 10.1083/jcb.90.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Hemmi K., Powers J. C. Reaction of serine proteases with substituted isocoumarins: discovery of 3,4-dichloroisocoumarin, a new general mechanism based serine protease inhibitor. Biochemistry. 1985 Apr 9;24(8):1831–1841. doi: 10.1021/bi00329a005. [DOI] [PubMed] [Google Scholar]
- James G. T. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal Biochem. 1978 Jun 1;86(2):574–579. doi: 10.1016/0003-2697(78)90784-4. [DOI] [PubMed] [Google Scholar]
- Keil B. Some newly characterized collagenases from procaryotes and lower eucaryotes. Mol Cell Biochem. 1979 Jan 26;23(2):87–108. doi: 10.1007/BF00226230. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Nordbring-Hertz B. Dialysis membrane technique for studying microbial interaction. Appl Environ Microbiol. 1983 Jan;45(1):290–293. doi: 10.1128/aem.45.1.290-293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North M. J. Comparative biochemistry of the proteinases of eucaryotic microorganisms. Microbiol Rev. 1982 Sep;46(3):308–340. doi: 10.1128/mr.46.3.308-340.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck S., Chase T., Rosenzweig W. D., Pramer D. Collagenase production by nematode-trapping fungi. Appl Environ Microbiol. 1980 Sep;40(3):567–570. doi: 10.1128/aem.40.3.567-570.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Leger R. J., Charnley A. K., Cooper R. M. Characterization of cuticle-degrading proteases produced by the entomopathogen Metarhizium anisopliae. Arch Biochem Biophys. 1987 Feb 15;253(1):221–232. doi: 10.1016/0003-9861(87)90655-2. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Nordbring-Hertz B., Harder W. An electron-microscopical analysis of capture and initial stages of penetration of nematodes by Arthrobotrys oligospora. Antonie Van Leeuwenhoek. 1985;51(4):385–398. doi: 10.1007/BF02275043. [DOI] [PubMed] [Google Scholar]