Abstract
Developmental cell fusion is found in germlines, muscles, bones, placentae, and stem cells. In Caenorhabditis elegans 300 somatic cells fuse during development. Although there is extensive information on the early intermediates of viral-induced and intracellular membrane fusion, little is known about late stages in membrane fusion. To dissect the pathway of cell fusion in C. elegans embryos, we use genetic and kinetic analyses using live-confocal and electron microscopy. We simultaneously monitor the rates of multiple cell fusions in developing embryos and find kinetically distinct stages of initiation and completion of membrane fusion in the epidermis. The stages of cell fusion are differentially blocked or retarded in eff-1 and idf-1 mutants. We generate kinetic cell fusion maps for embryos grown at different temperatures. Different sides of the same cell differ in their fusogenicity: the left and right membrane domains are fusion-incompetent, whereas the anterior and posterior membrane domains fuse with autonomous kinetics in embryos. All but one cell pair can initiate the formation of the largest syncytium. The first cell fusion does not trigger a wave of orderly fusions in either direction. Ultrastructural studies show that epidermal syncytiogenesis require eff-1 activities to initiate and expand membrane merger.
INTRODUCTION
Cell fusion is a ubiquitous and highly controlled process in eukaryotes. Developmental cell fusion is vital for mating and fertilization in yeast and humans, respectively. Cell fusion is required for the formation and maintenance of the muscular-skeletal system in vertebrates, in muscle fibers in Drosophila, and in nearly one third of all cells in Caenorhabditis elegans (Podbilewicz and White, 1994; Heiman and Walter, 2000; Wassarman et al., 2001; Abmayr et al., 2003; Shemer and Podbilewicz, 2003; Chen and Olson, 2005). In C. elegans, diverse cell fusions essential for organ formation and cell fate determination have recently become paradigms for developmental cell fusion (Podbilewicz and White, 1994; Mohler et al., 1998; Nguyen et al., 1999; Sharma-Kishore et al., 1999; Podbilewicz, 2000; Alper and Kenyon, 2002; Mohler et al., 2002; Shemer and Podbilewicz, 2002, 2003; Witze and Rothman, 2002). Cell fusion functions to sculpt organs and to accomplish defined body shapes (Sharma-Kishore et al., 1999; Witze and Rothman, 2002; Shemer and Podbilewicz, 2003). eff-1 (epithelial fusion failure) was identified as a gene encoding type I membrane proteins required for diverse epithelial cell fusion reactions. Mutations in eff-1 result in failure of epithelial cell fusion and developmental defects in organs where cell fusion normally occurs (Mohler et al., 2002; Shemer, 2002; Shemer and Podbilewicz, 2002). The activity of eff-1 is strongly regulated by homeobox containing genes (Shemer and Podbilewicz, 2002, 2003; Cassata et al., 2005). Other transcription and signaling factors have been shown to control the cell fusion process in C. elegans (Nilsson et al., 1998; Ch'ng and Kenyon, 1999; Shemer et al., 2000; Alper and Kenyon, 2001; Chen and Han, 2001; Koh and Rothman, 2001; Alper and Kenyon, 2002; Koh et al., 2002; Zhao et al., 2002; Shemer and Podbilewicz, 2003). The specific roles of different proteins that mediate and control cell fusion and the pathway of this fusion reaction remain unexplored. eff-1 induces cell fusion at distinct developmental times in different organs. Expression of eff-1 using a heat-shock promoter results in cell fusion between different cell types (Shemer et al., 2004; del Campo et al., 2005).
Interestingly, genes homologous to eff-1 have not been identified in Drosophila or vertebrates (Shemer and Podbilewicz, 2003; Podbilewicz and Chernomordik, 2005; Podbilewicz, 2006; Podbilewicz et al., 2006). Genetic screens in Drosophila have identified several genes required for myoblast fusion. Using elegant ultrastructural and developmental studies, it has been determined that the steps affected by these mutants include myoblast differentiation, acquirement of fusion competence, and recognition and adhesion between myoblasts (Doberstein et al., 1997; Abmayr et al., 2003; Chen et al., 2003). However, genes necessary and sufficient for the actual merger of two plasma membranes into one have not been reported in other developmental cell fusion reactions outside syncytin-mediated fusion between human trophoblasts (Mi et al., 2000) and eff-1–mediated cell fusion (Shemer et al., 2004; del Campo et al., 2005; Podbilewicz, 2006; Podbilewicz et al., 2006).
Here we hypothesized that since embryonic cell divisions in C. elegans are tightly controlled and invariant (Sulston et al., 1983), we may also find an ordered pattern of cell–cell fusions within the same large syncytium that will be nearly constant between individuals. We apply experimental approaches used for model fusion reactions (Stegmann et al., 1990; Phalen and Kielian, 1991; Frey et al., 1995; Hoekstra et al., 2002; Blumenthal et al., 2003; Chernomordik and Kozlov, 2003; Gibbons et al., 2003; Hu et al., 2003; Jahn et al., 2003; Bonifacino and Glick, 2004; McInerney et al., 2004) to address this hypothesis in living C. elegans. We analyzed cell fusion kinetics in developing embryos and dissected cell membrane fusion into defined stages. Surprisingly, we demonstrate that in the embryonic epidermis of C. elegans a variable cell fuses first, and for each fusogenic cell the anterior and posterior membrane domains fuse independently and asymmetrically. In addition, we found that stable intermediates in late stages of epidermal syncytia formation can be found in larvae and adults of partial loss-of-function eff-1 mutants. Thus, we show that eff-1 is required to initiate, expand and complete syncytia formation in the epidermis.
MATERIALS AND METHODS
Time-Lapse Multifocal Temperature-controlled Confocal Microscopy
Time-lapse movies were recorded using a Nikon Eclipse E-800 with a 60×/1.40 Plan Apo objective using a Bio-Rad MRC1024 confocal microscope with a custom-made copper stage and objective jacket for precise temperature control as described before (Rabin and Podbilewicz, 2000). The resolution of our confocal microscope is ∼250 nm. We used and show projections (flattened images) for each time point in all kinetic analyses. Four-dimensional stacks were also analyzed and archives of the complete original data sets are available for further analyses.
The cells responsible for the elongation of the embryo are the hypodermal cells (Sulston et al., 1983; Priess and Hirsh, 1986) and we measured the elongation of the lateral seam cells of the head (Rabin and Podbilewicz, 2000). The whole body elongation rate is 2.5-fold faster than the head elongation rate (Priess and Hirsh, 1986).
The largest syncytium (hyp7) is initiated in the embryo during elongation, and the events occurring from the comma stage to the 1.5–fold stage have been characterized here (Figures 1 and 2). During this time window the wild-type embryo does not move, allowing us to follow the kinetics as described below.
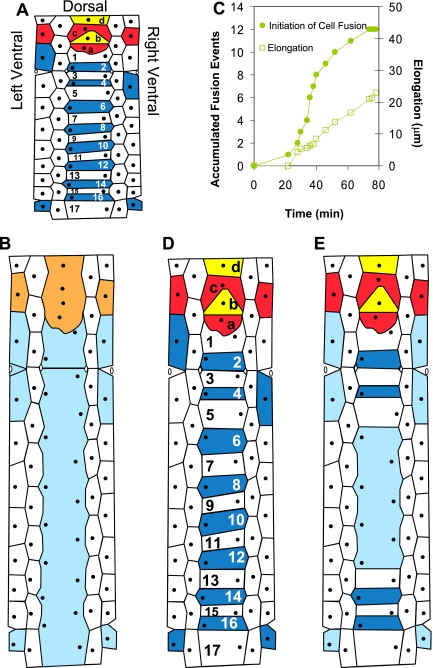
Figure 1.
Fusion between dorsal epithelial cells and elongation in C. elegans embryos. (A) Cylindrical projection of comma stage embryo before elongation and cell fusion. The dorsal epithelial cells are numbered so apical junctions (AJs) are readily identified. For example, junction 7/8 refers to AJ between cells 7 and 8. Red and yellow: dorsal cells (a–d) will fuse to form hyp6 syncytium (orange). Blue: dorsal cells (1–17) that will form hyp7 syncytium (light blue). Black circles, nuclei. (A, B, D, and E) Anterior is to the top of page. (B) A wild-type embryo elongates to the twofold stage and hypodermal cells fuse. (C) Kinetics of multiple cell fusion events and embryonic elongation. The number of accumulated fusion events in the dorsal hypodermis (•) and the increase in body length (□) for the same wild-type embryo are shown. Developing embryo expressing AJM-1::GFP was recorded at 23.1°C. A fusion event is defined as appearance of detectable AJ discontinuity. (D) Wild-type embryo (∼9°C) or eff-1(−) (15–25°C) with epidermal fusion failure. (E) idf-1(zu316) embryo (20°C) arrested at twofold stage of elongation with irregular dorsal fusions (Idf). Only some junctions fuse (light blue). Note that head and tail are not shown in cylindrical projections.
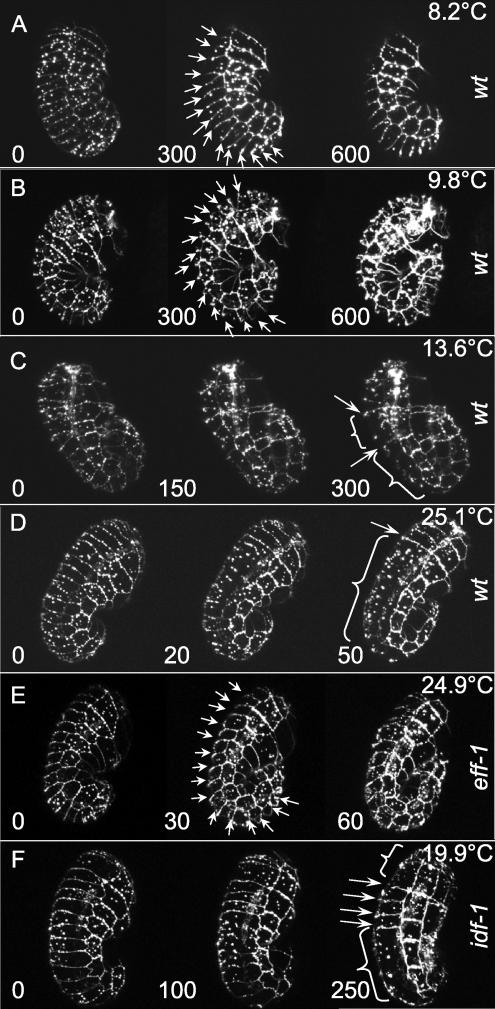
Figure 2.
In vivo cell fusion monitored by staining of AJs using AJM-1::GFP. Wild-type (A–D);. eff-1(hy21) (E); and idf-1(zu316) mutant (F). The numbers on left corners of each image show time in minutes after the comma stage (t = 0). (A) At 8.2°C both dorsal fusions and elongation of the embryo are blocked (see Supplementary Movie S1). (B) At 9.8°C dorsal fusions are blocked but elongation is observed (Supplementary Movie S2). (C) At 13.6°C some dorsal fusions are observed along with embryo elongation (Supplementary Movie S3). (D) At 25.1°C normal dorsal fusions are observed along with embryonic elongation. This is the phenotype that results in optimal embryonic development (Supplementary Movie S8). (E) eff-1(−) at 24.9°C. No dorsal fusion with normal embryonic elongation is observed (Supplementary Movie S10). (F) idf-1(−) at 19.9°C. Irregular dorsal fusions along with elongation are observed (Supplementary Movie S9). The temperatures are average ±0.5°C. Eggs are ∼50 μm long. (A–C and E) Lateral views; dorsal is left. (D and F) Dorsolateral views; anterior is up. Arrows, unfused junctions; brackets, fused regions.
For immunofluorescence we fixed and stained embryos using the methanol/acetone on dry ice protocol (Podbilewicz and White, 1994).
Kinetics of Cell Fusion: The Normal Sweep Method
We developed a semiautomated method to monitor membrane fusion, leading to disappearance of GFP-marked cell junctions in a single C. elegans embryo (Figure 3 and Supplemental Materials and Methods). Using the semiautomated normal sweep method, we could quantify the membrane fusion for each junction as follows:
 |
Thus, using this equation for images taken at regular time intervals for each embryo, we obtained the real-time kinetics of cell–cell fusion for multiple junctions in a single embryo. Note that for most of the embryos observed, there is excessive image noise above cell number 4 (as defined in Figure 1A; dorsal cells are numbered in anterior to posterior direction) and for nearly all the embryos, cells beyond number 12 gradually vanish from the dorsal view because of elongation. Therefore, to compare the same multiple fusion events in different embryos, we monitored the kinetics of fusion from junctions between cells 5/6 to cells 10/11 (Figure 3D).
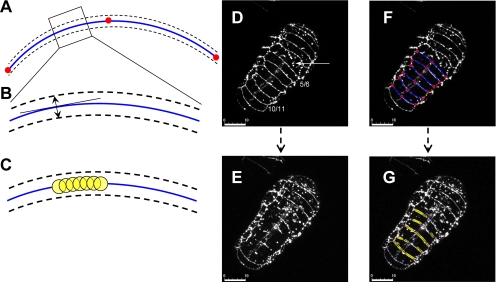
Figure 3.
Semiautomated normal sweep method to follow cell fusion in vivo. (A) Each junction is defined by an arc shown by the blue line, passing through three manually determined points (in red) in the dorsal view for each frame. The points need to be defined manually because the embryos twitch, move, and elongate, leading to deviations in locations of the same junction in different frames. (B) We look at 2–4 pixels above and below each point on the arc, along the normal drawn from the center of the circle to that particular point on the arc, to quantify presence or absence of fusion as described in experimental procedures section. (C) The progression of fusion is shown by drawing yellow circles that correspond to each point on the arc where the pixels are scored as blank (see Supplementary Material). (D) Junctions 5/6–10/11 in the dorsal view of an embryo. Note that one of the junctions above 5/6 is already partially fused (see arrow) in this embryo. (E) Disappearance of junctions 5/6–10/11 due to fusion. (F) Modeling the embryo junctions of interest as arcs. (G) Quantifying progression of fusion. Note the excessive image noise toward the anterior part of the embryo (above junctions 5/6). This image noise was observed in most of the embryos we imaged. Therefore, for consistent and proper comparison between different embryos we compared the fusion between junctions 5/6–10/11. Bars, 10 μm.
All analyses were done using MATLAB (MathWorks). The m-files are available upon request.
Quantifying the Kinetics of Cell–Cell Fusion
As shown in Figure 3A, first we defined the junctions (blue lines) as circular arcs fitted through three points (red circles defined roughly along the dorsal midline and at the edges of the cells in the dorsal view) that were determined manually for each junction in each individual frame. This is based on the simple mathematical concept that a unique circle passes through every three noncollinear points. Thus, the arc defining each junction was a part of unique circle passing through manually defined points. Because it is impossible to assign fixed geometry to a live biological specimen, especially when monitoring it in real time, each frame had its own set of arcs characterizing the individual junctions of the embryo. Knowing the total number of pixels along each arc (junction), we measured the apical junction (AJ) discontinuity, representing expanding fusion pores, by looking at appearance of blank pixels (pixel value < threshold; see below), as shown in Figure 3, B and C, by yellow circles along the arcs (blue lines). Because of junctions in live embryos not following strict circular-arc geometries, for each pixel coordinate along each arc we applied a normal sweep (depending on how good the “blue” arc described a junction by eye): a pixel is scored as blank (i.e., shown in yellow, Figure 3) only if 2–4 pixels above it and below it along the normal from the center of the circle corresponding to that arc and if the pixel value itself is lower than the threshold. Figure 3B shows the normal along a single point on the arc, which is always perpendicular to the tangent at that point on the arc. The threshold was uniformly selected for each junction for all frames as being 1–2 times the average intensity of the whole image, depending on the brightness of the junction (because all the junctions do not have the same GFP intensity). Figure 3C shows the “initiated macrofusion” represented by the yellow circles in absence of the blue line (junction). Supplementary Figure S1 shows four frames from a movie of a developing embryo in which yellow circles are seen to appear as the junctions “dissolve.”
Using our normal sweep method, we found that each fusion process for different junctions in different embryos grown at different temperatures follows sigmoidal kinetics (see examples in Figures 4 and Supplementary Figure S2). To extract the kinetic parameters of the curves we defined the fusion onset, i.e., the lag time (t1) and the time required to reach the sigmoidal saturation (t2). From these, the time required for the termination of the fusion event after the onset was determined as the macrofusion time (t2 − t1). This kinetic parameterization for understanding sigmoidal curves was introduced and has been explained in detail (Mittal et al., 2003).
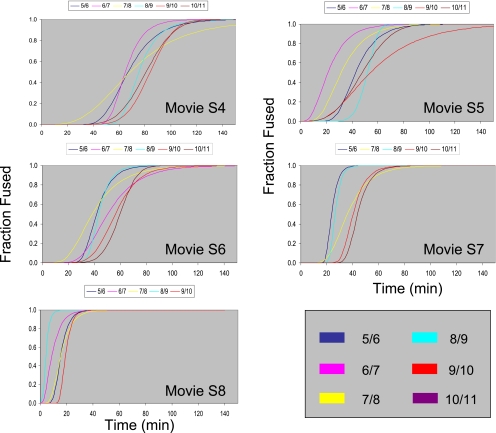
Figure 4.
Kinetic map of dorsal cell fusions in developing C. elegans embryos. The curves shown are sigmoidal fits to the kinetic data for the fusing junctions in different wild-type embryos grown and imaged at different temperatures (see Supplementary Movies and Supplementary Table S1). Supplementary Movies: S4, 16.8°C; S5, 20.5°C; S6, 23.1°C; S7, 23.7°C; and S8, 25.1°C. This figure highlights that using the semiautomated method we simultaneously characterize single cell–cell fusion events for multiple junctions of live embryos. Each panel represents a single embryo and the curves represent the “kinetic map” of hypodermal cell fusions.
Cell Fusion Analysis (Manual Method)
We measured the length of the discontinuity of the AJ between individual pairs of fusing cells over time and obtained the fusion rate at different temperatures (n = 43 cell pairs). The simple kinetic analyses described above were done by manually measuring the estimated length of fusion zone cross section from time-lapse movies obtained by confocal microscopy using NIH Image software over time. The linear distances were plotted against time, and a straight line was fitted. The slopes were then used to estimate the rates of disappearance of AJM-1::GFP staining. These rates varied from ∼50 nm/min at 11°C to ∼1000 nm/min at 25°C. The Arrhenius plot obtained by this method gave a slope used to estimate an apparent activation energy of 29.8 kcal/mol (r2 = 0.9474).
Electron Microscopy
Transmission electron microscopy was performed as described on fourth larval stage and adult eff-1(hy21ts) mutant animals grown at the semipermissive temperature 20–23°C (Shemer et al., 2004). Two tests were performed to determine whether a candidate microfusion site is indeed a cytoplasmic bridge that may have resulted from an incomplete membrane fusion event. First, the specimen was tilted in the transmission electron microscope (TEM) by ±10° to sharpen up the view of the plasma membrane bordering an apparent cell bridge, further tilting by ±50° showed whether the cell bridge is real. That is to say, if even when the specimen was tilted to extreme angles, we did not find an intact plasma membrane blocking the bridge, this was recorded as a bona fide cytoplasmic bridge. The second test was based on reconstructions in serial sections as previously described (Nguyen et al., 1999). In many instances we found apparently good bridges that failed to meet these criteria.
RESULTS
Experimental Design To Study Syncytiogenesis
To identify and study fusion intermediates during embryonic development and to uncover the kinetics of cell fusion in the hypodermis (epidermis) of C. elegans, we imaged transgenic embryos expressing AJM-1–green fluorescent protein (GFP) on the AJ of these epithelial cells (Knust and Bossinger, 2002). The dynamic behavior of AJs in C. elegans is a reliable assay for cell fusion of epithelial cells (Kenyon, 1986; Priess and Hirsh, 1986; Baird et al., 1991; Podbilewicz and White, 1994; Mohler et al., 1998, 2002; Nguyen et al., 1999; Sharma-Kishore et al., 1999; Alper and Kenyon, 2002). To follow cell fusion by confocal microscopy, we recorded time lapse 4D movies and followed the disappearance of the AJM-1::GFP reporter from the AJ between fusing cells (Hird and White, 1993; Mohler et al., 1998; Rabin and Podbilewicz, 2000; Koppen et al., 2001; Figure 1, A and B, Supplementary Movies S1–S10). Loss of the cell contact zone upon fusion was also followed using immunofluorescence and immunoelectron microscopy with the mAb MH27 that recognizes both endogenous AJM-1 and transgenic AJM-1::GFP proteins (Francis and Waterston, 1991; Koppen et al., 2001). In addition, cytoplasmic content mixing and membrane loss precede AJ disappearance (Mohler et al., 1998, 2002; Shemer et al., 2004), validating AJM-1::GFP loss as an assay for cell–cell fusion in C. elegans.
To quantitatively analyze cell fusion during elongation of C. elegans embryos, we determined the number of cell pairs that initiated fusion over time. We found a gradual increase in the accumulated number of cells with detectable disruption of AJ continuity. Figure 1C shows the time course for initiation of cell fusion events in a single elongating embryo incubated and imaged at 23°C (see also Figure 2 and Supplementary Movies). Embryonic elongation in C. elegans is characterized by defined stages starting with a comma shape (∼50-μm length; time = 0 in Figure 2) stage through 1.5-, 2-, 3-, and 4-fold (∼200-μm length) stages of elongation (Sulston et al., 1983; Priess and Hirsh, 1986). Most embryonic epidermal cell fusion events occur from comma to twofold stages (Podbilewicz and White, 1994).
idf-1 Mutant and Low Temperatures Block Cell Fusion
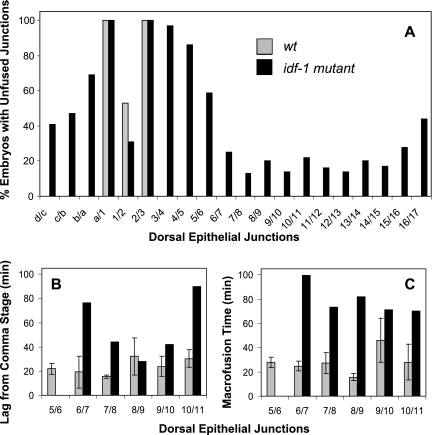
idf-1(zu316), an embryonic lethal mutant, was isolated in a screen for elongation defective embryos (Costa and Priess, personal communication). We found immunostained idf-1(zu316)–arrested embryos to have fewer dorsal fusions than in wild-type using the MH27 mAb. We genetically mapped idf-1(zu316) to the left arm of chromosome X and found that idf-1 acts recessively. Mutant embryos arrest between the 1.5–3-fold stage of elongation with fewer cell fusions in the dorsal hypodermal cells and posterior defects (Figure 1E; n > 1000). To test whether idf-1(zu316) is a hypomorph or a knockout, we constructed an idf-1 over deficiency (deletion) strain and found that the arrested idf-1(zu316)/deficiency embryos arrested earlier than the homozygous idf-1(zu316) embryos (data not shown). Thus, idf-1(zu316) is a hypomorph. The molecular identity of idf-1 has not been determined. Irregular cell fusion events occurring in idf-1 mutants were retarded compared with the timing of expected fusion events in wild type. idf-1(−) cells fused after the embryos began twitching and not before activation of the body wall muscle contraction (n > 100; Supplementary Movie S9). When comparing the dorsal cell fusion defects between different idf-1(−) arrested embryos we found that cells in the anterior dorsal hypodermis have a higher probability of remaining unfused than cells in the posterior hypodermis (Figures 1E, 2F, and 7A). However, all 21 dorsal epithelial cells that normally form the two major syncytia, hyp6 and hyp7 (Figure 1), are able to express the Idf phenotype (Figure 7A), suggesting that idf-1 activity is involved as part of the dorsal fusion machinery or in its regulation and not as a regional regulator of cell fusion affecting specific cells along the anterior-posterior axis.
Figure 7.
Characterization of the cell fusion defective phenotypes in idf-1(zu316) mutant. (A) Frequencies of dorsal hypodermal unfused junctions in idf-1(−) homozygous embryos (n = 64; ■) compared with wild type embryos (n = 15; ▩) at similar elongation stages at 15°C: 1.88 ± 0.14- and 1.89 ± 0.22-fold elongation for wild type and idf-1(zu316), respectively. Cells a–d are part of hyp6; cells 1–17 are part of hyp7 (Figure 1A). There is high variability in the dorsal unfused pattern, although the occurrence of unfused junctions is higher at the anterior part of hyp7; these results may imply that idf-1 is crucially required for proper fusion in these cells. (B and C) Kinetics of cell fusion in idf-1 embryos is defective as seen for both the lag and macrofusion times. Note that our single event kinetic measurements were done on an idf-1 embryo for which 5/6 did not fuse (■). These embryos were imaged at 20 ± 0.5°C. Wild-type embryos were triplicates (▩).
Low temperatures have been used to stabilize and identify important steps in viral fusion (Stegmann et al., 1990; Schoch et al., 1992; Chernomordik et al., 1998; Melikyan et al., 2000). To determine the effects of temperature on embryonic epidermal cell fusion in AJM-1::GFP embryos, we incubated comma stage embryos (time = 0; Figure 2) at different temperatures and recorded the changes in dorsal epidermal AJs upon fusion. Neither cell fusion nor embryonic elongation was observed at temperatures below 8.5°C, even after incubation for 24 h (n = 11; Figure 2A). Wild-type embryos incubated between 10 and 25°C reached the twofold stage (halfway through elongation) with most of the dorsal cells fused (n > 100; Figure 2, C and D). We found that cells failed to fuse in wild-type embryos grown at 8.5–10°C; these animals reached the twofold stage of elongation and the muscles twitched demonstrating that the embryos with cells that fail to fuse have some physiological activities (n = 19; Figure 2B).
To compare the “frozen” fusion phenotype obtained at ∼9°C to the cell fusion defects obtained in eff-1 and idf-1 mutants, we imaged embryonic elongation in eff-1(hy21) mutant embryos expressing AJM-1::GFP. In eff-1(hy21) embryos, dorsal epidermal cells completely failed to fuse at 15 and 25°C (Figures 1D and 2E). Although eff-1(−) embryos elongate and remain dumpy (short and fat) with bulged tail, lumpy body, and other morphological defects maintained during postembryonic development (Mohler et al., 2002; Shemer, 2002), wild-type embryos elongating at ∼9°C irreversibly arrest at the twofold stage. It seems that elongation is not dependent upon cell fusion, because eff-1 blocks cell fusion but not elongation. It appears that other defects unrelated to epithelial fusion failure may be responsible for the embryonic arrest observed in “frozen” and idf-1(−) embryos (e.g., microtubule depolymerization in the cold).
In summary, we can block cell fusion in wild-type cells at ∼9°C partially phenocopying eff-1 and idf-1 mutant embryonic cells.
Kinetics and Temperature Dependence of Cell Fusion In Vivo
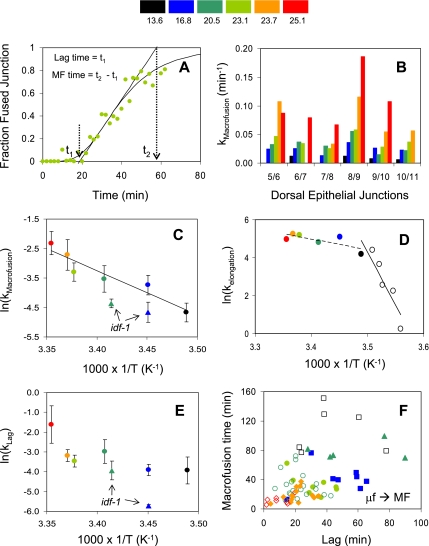
To study intermediates of cell fusion we initiated a kinetic approach in the epidermis of the embryo. For a detailed characterization of cell fusion kinetics, we developed a computer-based method where cell fusion was measured by following the loss of AJ as the appearance of blank pixels in each junction and for each individual frame (see Materials and Methods; Figure 3 and Supplementary Figure S1). Using this semiautomated method, we found that each fusion process for six to nine distinct junctions, in embryos grown at different temperatures, follows sigmoidal kinetics (n = 74 cell pairs; Figures 4 and 5A and Supplementary Figure S2). To extract the kinetic parameters of the curves we defined the delay time or lag (t1) and the time required to reach the sigmoidal saturation (t2). From these, the time required for the termination of the fusion event after the onset was determined and defined as the macrofusion time (t2 − t1). This novel semiautomated method was validated by a different manual method to measure macrofusion (see Materials and Methods). Using the semiautomated method, we found that at the fusion-permissive temperatures the macrofusion rate [kMacrofusion = 1/(t2 − t1)] for each cell pair increased with temperature (Figure 5B). We have analyzed 5–6 pairs of fusing cells in 10 different embryos grown from 13 to 25°C (Figure 4).
Figure 5.
Kinetics of cell fusion events in C. elegans embryos. (A) Solid symbols show kinetics of a single dorsal fusion event measured using the Normal Sweep Method. Solid line shows a theoretical fit to the observed sigmoidal data (see Supplementary Material). To extract the kinetic parameters of the curves we defined lag time for fusion onset (t1) and time required to reach the sigmoidal saturation (t2). From these, Macrofusion (MF) time of the fusion event after onset was determined: (t2 − t1); Temperature 23.1°C, junction 7/8, Supplementary Movie S6. (B) Temperature dependence of cell fusion for each junction (a color code is defined from black to red with increasing temperature; A–F). From the macrofusion times we obtained the rate constants, given by 1/(t2 − t1), for different junctions (numbers as described in Figure 1A). Junction 8/9 had the fastest macrofusion rate at all temperatures tested. (C) Arrhenius plot for macrofusion rates for junctions 5/6–10/11. Each point represents a single embryo, and error bars are due to multiple junctions. The apparent energy of activation was estimated from the logarithmic form of the Arrhenius equation: ln(k) = lnA−E*/(RT). k is the rate of the reaction, E* is the effective activation energy, T is the absolute temperature (in °K), A is a pre-exponential factor, and R is the gas constant (1.9872 calories/°K · mol). ln(k) versus 1/T was used to estimate the apparent energy of activation of the reaction (E* = ∼30 Kcal/mol) from the slope of the Arrhenius plot. Green triangle, idf-1(−) at 19.9°C. Blue triangle, idf-1(−) at 16.8°C. (D) Arrhenius plot for elongation rates of embryos measured at different temperatures (open symbols for 9–13°C; Rabin and Podbilewicz, 2000). At higher temperatures, elongation does not appear to depend on temperature, whereas at lower temperatures, slow elongation has an apparent activation energy of ∼109 kcal/mol based on the slope of the fitted line shown (r2 = 0.8269). (E) Arrhenius plot of the lag does not show a clear linearity. This suggests that lags may involve multiple steps, rather than a single rate-limiting step. Green triangle, idf-1(−) at 19.9°C. Blue triangle, idf-1(−) at 16.8°C (with SD = 0.08, not shown). (F) Macrofusion times are plotted versus lag for each junction at each of the temperatures monitored. The lack of correlation indicates that there are at least two distinct mechanistic steps in the reaction: microfusion (μf), which precedes the onset of the first dorsal fusion detectable by our method, and Macrofusion (MF) that is measured by our normal sweep method. Black empty squares, wild-type embryo at 13.6°C. Other colors are for different junctions according to the temperature scale.
To quantify the temperature dependence of developmental cell fusion, we have used conventional Arrhenius plots in which the slope characterizes the apparent activation energy of the rate-limiting step of the process. To obtain an Arrhenius plot, we used the macrofusion rates from Figure 5B. The average macrofusion rates were calculated for each embryo (a total of 6 embryos, 32 junctions). The temperature dependence of the macrofusion rates over the range 13–25°C is linear in a semilog plot with a regression coefficient of ∼0.91; a similar linear slope was independently obtained using a different method to estimate the macrofusion rates over the range of 11–25°C (Figure 5C; see Materials and Methods).
To determine whether our kinetic analyses of cell fusion in vivo have a distinct behavior from other temperature-sensitive processes, we measured the rate of embryonic elongation at different temperatures using the same embryos where we measured the cell fusion rate. The initial rates of embryonic elongation changed from 1.3 nm/min at 8.2°C to 193.5 nm/min at 24°C (Rabin and Podbilewicz, 2000). The temperature dependencies of embryonic elongation have different slopes in different temperature ranges (Figure 5D). This is in contrast to a single apparent rate-limiting step for cell fusion in C. elegans embryonic hypodermis (Figure 5C), implying, along with the cold block of cell fusion (Figure 2B), that cell fusion and embryonic elongation are two distinct processes (see Discussion).
Kinetic Studies Reveal at Least Three Steps of Cell Fusion
Is fusion pore expansion (macrofusion) simply a continuation of the same processes that operate during the lag time? If this were the case, then one would expect the lag time to directly correlate with the macrofusion time. To test this, we plotted macrofusion versus lag times for each junction. For all temperatures studied we found no correlation between lag and macrofusion time, indicating that these are independent kinetic steps (Figure 5F). Moreover, the Arrhenius plot of the lag rates gave a very weak trend (Figure 5E) compared with macrofusion rates (Figure 5C), supporting the interpretation that the lag and macrofusion stages are different mechanistic steps in the process. Because lag and macrofusion are distinct stages, we infer a new stage that occurs during lag time. This microfusion stage cannot be resolved using live confocal microscopy of AJ disappearance. Thus, based on kinetics we have been able to dissect cell fusion into two distinct steps: an early step of microfusion followed by a stage of expanding gap or macrofusion, which we actually measure in our assay.
In various membrane fusion studies, the lag time has proved to be a very significant measurement to investigate when the system is “ready” to fuse (Stegmann et al., 1990; Bron et al., 1993; Danieli et al., 1996; Munoz-Barroso et al., 1998; Parlati et al., 1999). To analyze the lag phase in our system, we pooled the lag time parameters into a cumulative distribution showing a fraction of events that had already occurred by a given time (Supplementary Figure S3). To explain this lag distribution, we investigated different linear kinetic models (see Supplementary Material). We found that the simplest model that fits the data includes two distinct steps. Taken together, this kinetic model and the finding that the Arrhenius plot for lag does not show a linear relationship as would be expected for a single rate-limiting step process (Figure 5E), implying that initiation of cell fusion in C. elegans is at least a two-step process.
In summary, kinetic dissection of cell fusion in C. elegans embryos shows that this is at least a three-step process: Two steps during the lag stage leading into microfusion and a third step for the actual gap expansion between cells (macrofusion) that results in syncytia formation.
idf-1 and eff-1 Genetic Interactions during the Epidermal Cell Fusion Process
To investigate whether idf-1 and eff-1 interact genetically, we constructed a strain to study the double mutant idf-1; eff-1 and compared the embryonic phenotypes of the single mutants with the double mutants at 15 and 20°C (see Materials and Methods). In wild-type embryos most dorsal and ventral fusions take place by the twofold stage of elongation. idf-1(−) embryos arrest with the characteristic Idf phenotype, namely, irregular dorsal fusions. eff-1(−) embryos elongate with neither dorsal nor ventral epithelial fusions, and double mutants idf-1(−); eff-1(−) arrest at the 1.5–3-fold stage of elongation without any cell fusion (Figure 6). At 15 and 20°C the phenotype of the double mutant is a combination of the individual Idf and Eff phenotypes (see Materials and Methods). The lethality associated with idf-1(−) may not be directly related to cell fusion defects, but rather, might represent an additional function for idf-1.
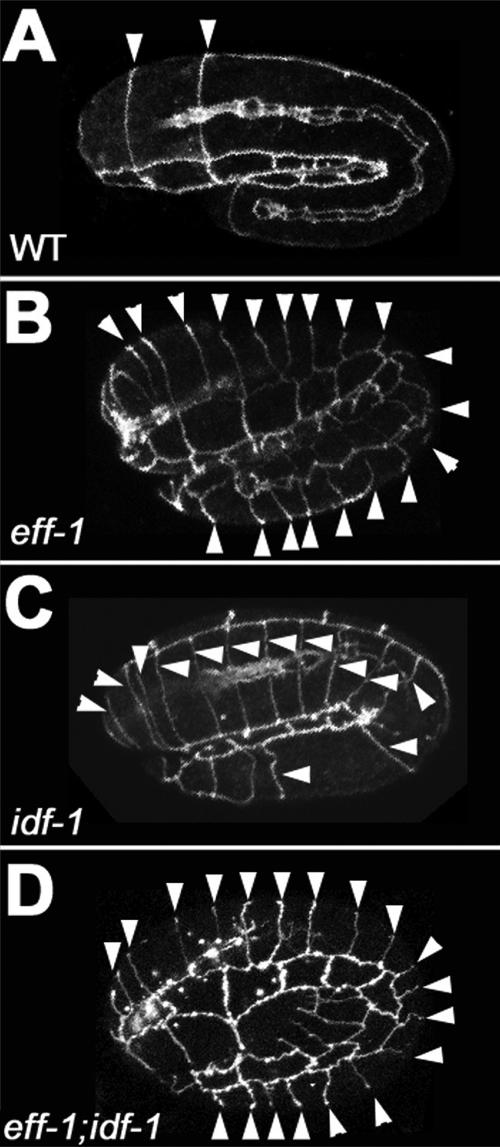
Figure 6.
Genetic interaction between idf-1 and eff-1 mutants. Confocal projections of representative wild-type, single, and double mutant embryos grown at 15°C. (A) Wild-type embryo, most epidermal fusions have occurred in the hypodermis. (B) eff-1(hy21) embryo with all junctions unfused. (C) idf-1–arrested embryo with some unfused dorsal cells. (D) Double mutant arrested with a round shape; the animal was moving and all hypodermis is unfused. Dorsal partial projections of the embryos are shown. Anterior is to the left. Arrowheads, unfused junctions. (A–C) MH27 immunofluorescence. (D) AJM-1::GFP background. Egg length, ∼50 μm.
To test whether there are cell differentiation changes in idf-1(−) embryos, we have stained mutant embryos with different tissue-specific monoclonal antibodies (e.g., intestine, pharynx, body wall muscles, lateral epidermis) and we have not found differences with wild-type except for the fusion failure in the dorsal hypodermis. In addition, DIC analyses of idf-1(−) embryos showed a twisted tail defect different from the Eff-1 tail phenotype (Gattegno, 2003). To test whether idf-1 and eff-1 genes function redundantly to promote fusion, we constructed and tested hsp::eff-1; idf-1/+; ajm-1::gfp animals. We obtained the ectopic fusion phenotype in homozygous idf-1(−) embryos after heat-shock (n = 14/84). Taken together, these results are consistent with idf-1 and eff-1 genes acting independently to positively control cell fusion. An alternative explanation is that idf-1 positively controls eff-1.
Comparative Kinetics of Cell Fusion between idf-1(−) and idf(+) Embryos
To understand the kinetics of cell fusion, it would be useful to have mutations that affect the kinetic parameters. Even the weakest allele of eff-1 has a complete failure in the initiation of embryonic cell fusion, so we could not use eff-1 in kinetic analyses. However, in idf-1(−) embryos some dorsal epidermal cells are able to fuse several hours after the normal time of fusion during early embryonic elongation (Figure 7A). We imaged nearly 100 embryos and analyzed the kinetics of 10 fusion events from 2 independent embryos that were optimal for quantification using the semiautomated method. We found that these embryos arrested at the twofold stage with characteristic Idf phenotypes (Figure 1E) showing 10 pairs of dorsal cells fusing (Supplementary Movie S9 and Figure 2F). These cell fusions followed characteristic sigmoidal behaviors (see example in Supplementary Figure S2), showing longer lag and macrofusion times than in wild-type embryos imaged at the same temperatures (Figure 7, B and C, and green and blue triangles in Figure 5, C and E). Lag and macrofusion times in idf(−) embryos were significantly longer for the fusing pairs of cells than for wild-type (see Supplementary Material).
Cell Fusion Pioneers Are Variable
The end result of the cell fusion process is the formation of functional multinucleate cells (syncytia). To define whether there are pioneer junctions (cell pairs) that consistently fuse to form binucleate cells, followed by nonpioneer cells that undergo secondary fusion to binucleate syncytia, we identified 10 junctions that start the formation of binucleate cells in different embryos (n = 10). We define pioneer pairs as any two adjacent cells that initiate cell fusion to each other before starting fusion with other cells. We found between one to two pioneers per embryo in the area that we could analyze consisting of dorsal cells 5–11, precursors to hyp7 (see Figure 1A; n = 63 junctions). Only junction 2/3, between cells 2 and 3, was not found to act as a pioneer (n > 1000). Each of the four junctions 6/7–9/10 were pioneers with a similar frequency (0.2–0.3; n = 10). This shows that syncytial precursors of the dorsal hypodermis fuse stochastically (see Figure 4). For each pioneer junction we define anterior and posterior neighboring nonpioneer junctions that fuse later. Pioneers can have the fastest (n = 3/10) or the slowest (n = 5/10) macrofusion rates compared with their nonpioneer anterior and posterior neighbors. The anterior cell that fuses to a pioneer syncytium was the fastest (n = 3/10) or slowest (n = 3/10) compared with pioneer and posterior nonpioneer cells. The posterior cell fused the fastest (n = 4/10) or slowest (n = 2/10), and in 7 of 10 cases it was the second fusion event in the syncytium. This last observation may explain the apparent anterior to posterior wave of fusion events (Podbilewicz and White, 1994; Mohler et al., 1998; Mohler et al., 2002).
In summary, most junctions in the dorsal hypodermis can initiate a syncytium with a pioneer fusion event. Additional nonpioneer cells fuse to the initial intermediate binucleate syncytium that expands into a giant cell. Our comparison between kinetic parameters of pioneers and nonpioneers indicates that fusion initiation at one side of the cell affects neither the lag time of microfusion nor the macrofusion rate at the other side of the same cell. Autonomous fusion for adjacent junctions argues against the hypothesis that the cell fusion pathway is driven by the lateral tension in the membrane bilayer that might be generated by osmotic effects or by cytoskeleton activity.
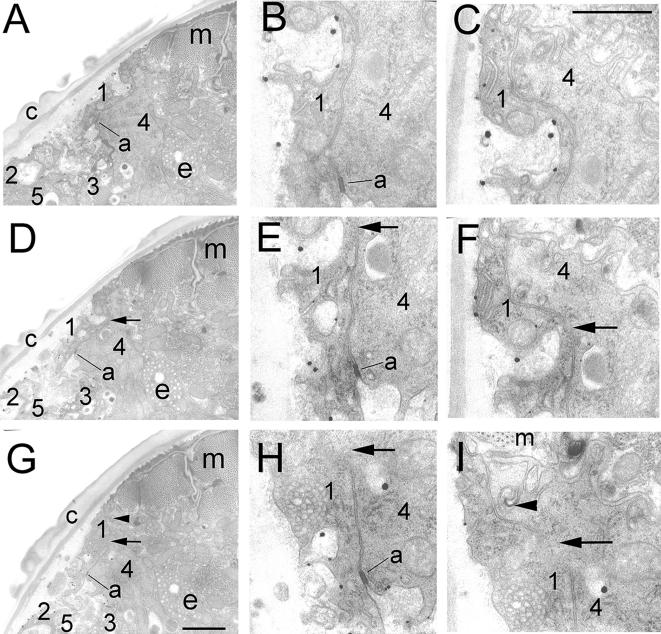
Microfusion: Ultrastructural Intermediate of EFF-1–mediated Epidermal Cell Fusion
TEM has been previously used to identify structural cell fusion intermediates in yeast, worms, flies, and mammals (Kalderon and Gilula, 1979; Baron et al., 1986; Doberstein et al., 1997; Gammie et al., 1998; Mohler et al., 1998; Heiman and Walter, 2000). In C. elegans, the existence of a distinct stage of microfusion in the fusion pathway is independently supported by kinetic analyses (see above) and the phenotypes in myoepithelial cells observed by TEM in eff-1 conditional mutants that were grown at the semipermissive temperature where microfusion intermediates failed to expand (Shemer et al., 2004). We looked for similar intermediates in the hypodermis of the same fixed and sectioned specimens and found some initiated fusion events that failed to expand (Figures 8 and 9). We conclude that EFF-1 in the hypodermis is required both to initiate cell fusion but also to expand membrane gaps of 20–50 nm to complete macrofusion of around 20,000 nm.
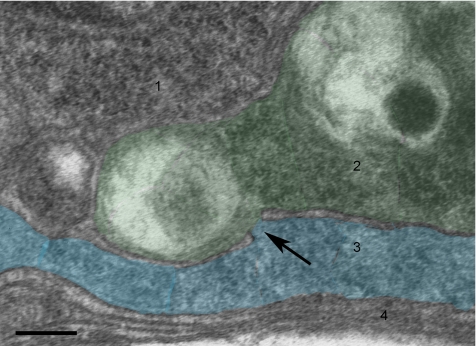
Figure 8.
TEM showing microfusion between hypodermal cells. Micrograph shows microfusion or gap of ∼25-nm diameter between two partially fused cells (arrow). Four hypodermal cells (1–4) were pseudocolored to show the borders. For more details see Materials and Methods and Supplementary Figure S4. Bar, 0.1 μm.
Figure 9.
Serial section TEMs of eff-1(hy21) animals with microfusions. (A, D, and G) Three serial sections show microfusion (arrows) between two hypodermal precursors in D and G. (B and C) Details of A show intact plasma membrane junctions. (E and F) Higher magnification of D show gap in the cell junctions between cells 1 and 4. (G) Partial fusion between cells 1 and 4. (H and I) Details of G with fusion intermediate. Arrowhead shows abnormal apical junction. a, apical junction; c, cuticle; 1–5, hypodermal cells; m, body wall muscles; e, excretory cell; arrow, microfusion; arrowhead, abnormal apical junction remnant. Bars, 1 μm (A, D, and G); 0.5 μm (B, C, E, F, H, and I).
DISCUSSION
Dissection of Cell Membrane Fusion into Three Defined Stages in Developing Embryos
Here, we have dissected the pathway of cell fusion during embryonic development of C. elegans. We developed a new system to simultaneously record, measure, and analyze individually fusing epidermal cells in live embryos. In contrast to studies on simpler fusion systems that investigate maximum pore sizes of a few nanometers (microfusion); here we measure the kinetics of large expanding gaps, of the order of hundreds of nanometers per micron (macrofusion), resulting from single cell–cell fusions critical for animal development. We have found that at these scales, each fusion event follows sigmoidal kinetics in wild-type and idf-1 mutant embryos. On the basis of the sigmoidal behavior, we define lag and macrofusion times as the kinetic parameters for each pair of fusing cells. We found that 9°C incubations block macrofusion but not embryonic elongation, and idf-1 mutations either block early cell fusion steps or slow down macrofusion rates. Moreover, here we found that in the epidermis of eff-1 mutants grown at the semipermissive temperature, there are stable microfusion intermediates similar to ultrastructural microfusions in eff-1–mediated muscle–muscle fusion (Shemer et al., 2004), strengthening the notion that during the lag phase there are two kinetic steps followed by expansion or macrofusion. It is conceivable that additional gene(s) may function in some cell fusion events in epidermal and myoepithelial cells of the pharynx, explaining the stable microfusions in eff-1 mutants (Shemer et al., 2004).
Genes and Kinetic Behavior Characteristic of Developmental Cell Fusion
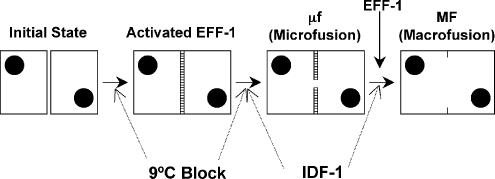
In Figure 10 we propose a model for the cell fusion process in epithelial cells and hypothesize the roles of eff-1 and idf-1 in specific stages based on our findings. Epidermal cell fusion in the embryo is dependent on the activity of EFF-1 (Mohler et al., 2002). Expression of EFF-1 is enough to fuse cells in C. elegans and activated EFF-1 primes the system for cell fusion (Shemer et al., 2004; del Campo et al., 2005). We identified three steps in the cell fusion pathway. Two steps in the lag may involve activation of EFF-1 and the initiation of cell fusion or microfusion. Recently we found that expression of EFF-1 on the surface of insect Sf9 cells is enough to fuse cells via hemifusion (Podbilewicz et al., 2006). The 9°C cell fusion block we observed in C. elegans embryos may be analogous to the 4°C block in influenza virus fusion that freezes the membranes in a hemifusion state (Chernomordik et al., 1998). The discontinuity in the plasma membranes has to expand and this expansion of the microfusion is what we measure in the cell macrofusion assay as the disappearance of the AJ. Although the early stages of membrane fusion are rapid (from fractions of a second to 1 or 2 min; Kaplan et al., 1991; Plonsky and Zimmerberg, 1996; Mohler et al., 1998; del Campo et al., 2005), the complete disappearance of the membranes and the apical junctions associated to them is a temperature-dependent process that takes several minutes (Figure 4).
Figure 10.
Working model for dorsal epithelial cell–cell fusion in developing C. elegans embryos. Kinetic modeling has shown a three-step kinetic process. The initial state has two cells adhering to each other. There is a transition from the initial state to a state where EFF-1 is “activated.” The activated protein(s) mediate microfusion. The kinetically defined lag time comprises two steps culminating in microfusion. Microfusion (μf) is transformed into Macrofusion (MF). Because no macrofusion is observed for the embryos at 9°C, this temperature may affect either, or both, of the first two steps. The idf-1 mutants show variable fusion events in embryos, apparently with both lag and macrofusion steps affected. Statistical analysis suggests that idf-1 mutants are affected in the lag and macrofusion steps (see Supplementary Material).
Although Arrhenius plot analysis is a convenient way to quantify the temperature-dependence of the process and to compare it with those of other processes, developmental cell fusion is undoubtedly a complex multistep process. The linearity of the Arrhenius plots suggests that the same process is rate limiting over the entire temperature range (Figure 5C). The slope of the plot suggests that this rate-limiting step of macrofusion has an apparent activation energy of 30 kcal/mol. The apparent activation energy of the macrofusion step, 30 kcal/mol, is close to apparent activation energies reported for viral fusion (34–42 kcal/mol), pore formation during exocytosis (23 kcal/mol), Ca2+-triggered exocytosis (27 kcal/mol), transferrin endocytosis (30–36 kcal/mol), and fusion of protein-free lipid bilayers (Iacopetta and Morgan, 1983; Clague et al., 1990; Oberhauser et al., 1992; Lee and Lentz, 1998; Earles et al., 2001).
idf-1 may affect the lag and the macrofusion stages (Figure 5, C and E). Macrofusion may involve vesiculation of the plasma membrane surrounding the microfusion gap. This is supported by TEM studies of embryonic (Mohler et al., 1998) and postembryonic (Nguyen et al., 1999) epidermal cells that have revealed the appearance of vesicles in the gap between fusing cells, suggesting that vesiculation after initial microfusion may be a critical step in syncytia formation and morphogenesis (Podbilewicz, 2000, 2006; Podbilewicz and Chernomordik, 2005).
Actin contraction may be the rate-limiting step for the actin-mediated elongation of C. elegans embryos (Priess and Hirsh, 1986; Costa et al., 1998; McKeown et al., 1998). Morphogenesis is strongly temperature dependent only between 8 and 15°C with an apparent activation energy of 110 kcal/mol (Figure 5D), similar to the apparent activation energy of F-actin movement on rabbit skeletal myosin (Anson, 1992). At temperatures in the range of 15–25°C, the rate of elongation is weakly temperature-dependent with an estimated apparent activation energy of 1.5 kcal/mol and an average elongation rate of 160 ± 23 nm/min. In contrast to elongation, the Arrhenius plots for the macrofusion step were linear at 13–25°C using the semiautomated method and at 11–25°C using the manual method, indicating that within this temperature range cell fusion in C. elegans has the same rate-limiting step.
Using stringent criteria including careful tiltings and reconstructions in serial sections (Materials and Methods), we found that microfusion events in eff-1 mutants do occur between epidermal cells that fail to complete syncytia formation. In multiple cases we were able to distinguish places where the membrane just twists into a new plane of section (detectable when tilting the TEM grid) from true microfusion sites where fields of cytoplasmic ribosomes span the gap across the cell border. Thus, although the unfused cells bear irregular and convoluted common borders not found in wild-type animals, we can confirm multiple microfusions that reveal another function for EFF-1 in the expansion from microfusion to complete fusion. There appear to be multiple chances for such microfusions to occur between the same hypodermal cells as their common borders are very extensive. Structurally similar microfusions have been identified in cells infected with human cytomegalovirus and measles virus (Firsching et al., 1999; Gerna et al., 2000; Ehrengruber et al., 2002). It is believed that viral induced microfusions may be involved in viral spreading. In addition, stable microfusion-like structures have been described between fibroblasts and cardiomyocytes (Driesen et al., 2005). Although in virus-cell fusion and intracellular membrane fusion a lot is known about the initial steps leading to fusion pore formation, relatively little is known about fusion pore expansion (Scepek et al., 1998; Dutch and Lamb, 2001; Haller et al., 2001; Gibbons et al., 2004; Jaiswal et al., 2004; Leikina et al., 2004; Nolan et al., 2006).
In summary, independent kinetic, genetic, and ultrastructural studies on cell fusion are consistent with at least three steps in the membrane fusion pathway in C. elegans, with eff-1 acting in early local fusion (microfusion) and late expansion (macrofusion) steps of the pathway.
Fusion Events Do Not Typically Occur Symmetrically
We have developed a sensitive and unique paradigm for studying kinetics of cell fusion in living embryos of C. elegans and show that it can be used as a model to analyze how molecules identified in genetic screens for cell fusion defective mutants affect specific steps in the dynamic process of cell fusion. Indeed, in a screen for mutants defective in embryonic morphogenesis (Costa and Priess, personal communication; Costa et al., 1998) idf-1(zu316) was identified, and here we show how this mutant gene slows down two distinct kinetic steps of cell fusion in vivo. Unexpectedly, idf-1 mutants affect some cell–cell fusions in a different manner, arbitrarily. Some cell pairs have a complete block, whereas others have a retarded initiation followed by a slow execution. This differential kinetic behavior of cell fusion in the same embryo may reveal intrinsic differences between cells and variability in the trigger of cell fusion in a developing tissue. One explanation for these results is that low density of active EFF-1 on the fusion sites may be sufficient to initiate pore formation but not for their expansion as shown for influenza virus fusion (Kozlov and Chernomordik, 2002; Chernomordik and Kozlov, 2003; Leikina et al., 2004). Alternatively, other genes may also be required to act along with EFF-1 to complete fusion. It is surprising that the arrest phenotype of the idf-1 mutant phenocopies the cold fusion block (9°C). idf-1 has a role in dorsal epidermal fusion and additional essential roles probably unrelated to cell fusion. Future TEM of idf-1–arrested embryos compared with the 9°C block, together with physiological tests to follow lipid and content mixing between fusing cells, should give us a better understanding of the intermediates in cell fusion and the specific roles of EFF-1 and IDF-1 in the process.
Membrane Domains Fuse Autonomously and Asymmetrically.
Early studies on epidermal cell fusion in C. elegans embryos suggested that there is a variable program in the sequence in which 23 syncytial precursor cells fuse to form the hyp7 syncytium. These studies were based on immunofluorescence of hundreds of fixed specimens at different stages in morphogenesis and reconstruction of the pathways of cell fusion during syncytiogenesis (Podbilewicz and White, 1994). More recently, using GFP reporter genes and membrane markers it was possible to follow syncytiogenesis of individual embryos in real time (Mohler et al., 1998, 2002; Shemer et al., 2004; del Campo et al., 2005). Taken together these studies showed that the final position, number, and identity of the cells that fuse is invariant during development though the fusion sequence is variable between individuals. In addition, cytoplasmic content mixing followed using GFP reporters is completed in 2–3 min, whereas the complete rearrangement of the plasma membranes and apical junctions takes about 40 min at 23°C (Mohler et al., 1998; Shemer et al., 2004; del Campo et al., 2005).
Here, for all fusion events in wild-type and the idf-1 mutant embryos, we observed sigmoidal kinetics of fusion and measured the characteristic parameters: Lag (microfusion) and macrofusion times. Each cell pair fuses with characteristic macrofusion rate in embryos monitored at different temperatures. It appears that the anterior membrane domain of a fusion competent cell fuses or fails to fuse independently of the posterior plasma membrane domain of the same cell. Thus, the anterior or posterior end can fuse faster that the opposite end of the cell, but which end fuses faster is random. Although the lateral membranes are fusion incompetent during embryogenesis in the wild-type, the anterior and posterior membranes develop their fusion competence autonomously and without any apparent symmetry. This control of the fusion competence of specific cells and membrane domains can be overruled by ectopic activity of EFF-1 in fusion-incompetent cells (Shemer et al., 2004). Ectopic expression of eff-1 followed by abnormal tissue-specific cell fusion can also be the result of inactivation of Engrailed/ceh-16–dependent transcriptional repression of eff-1 in lateral seam cells (Cassata et al., 2005), inactivation of vacuolar ATPase in the lateral hypodermis (Kontani et al., 2005), or inactivation of lin-39/Deformed repression of eff-1 in the ventral vulval precursor cells (Shemer and Podbilewicz, 2002).
Founder Cell Fusion Event Is Variable.
Most dorsal precursor cells of the hyp7 syncytium, except one structural junction, are competent to be pioneers or founder cells. Macrofusion and microfusion rates are not correlated with pioneer and nonpioneer cells having similar probabilities to fuse with the fastest or slowest macrofusion rates. These findings show that there is randomness in the initiation and completion of a genetically programmed sequence of cell fusion events. Localization of EFF-1 in the cell–cell contact zone above a certain threshold may explain these apparently stochastic events (del Campo et al., 2005). The concept of a stochastic epidermal founder cell in C. elegans described here is analogous to the founder myoblasts and fusion-competent myoblasts hypothesis in Drosophila (Rushton et al., 1995; Abmayr et al., 2003; Chen et al., 2003; Englund et al., 2003). However, our definition of the founder cell is strictly kinetic with respect to the first detectable cell fusion event that occurs after cell fate determination, migration, recognition, adhesion, differentiation, and patterning of the epidermis. In contrast, in the muscles of Drosophila, founder cells are pioneers for myogenesis that differ from fusion-competent myoblasts primarily by distinct phenotypes of mutations and differential expression of molecular markers required for recognition, signaling, adhesion, patterning, differentiation, and fusion competence. In contrast to myoblast fusion in Drosophila, in the epidermis of C. elegans, tightly regulated homotypic expression of EFF-1 initiates and expands cell fusion (Podbilewicz et al., 2006).
In summary, here we have used a new system to study the molecular and cellular mechanisms of cell membrane fusion in developing animals and showed how mutations, temperature blocks, ultrastructural, and kinetic analyses reveal that the first cell fusion event is variable and that membrane fusion events have independent and asymmetric anteroposterior kinetics. Moreover, eff-1 activity is required at early and late stages of the process of epidermal syncytiogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Costa and J. Priess for kindly providing idf-1(zu316); J. Simske for ajm-1::gfp; C. Ramos, E. Leikina, H. Delanoe, E. Zaitseva, M. Glickman, J. Zimmerberg, Y. Rabin, S. Hess, P. Blank, I. Kolotuev, N. Assaf, L. Broday, and M. Suissa for discussions; M. Krause, S. Vogel, M. Kozlov, Y. Gruenbaum, K. Melikov, S. Joshua, and G. Shemer for critically reading the manuscript. This work was supported by grants from Israel Science Foundation, The Charles H. Revson Foundation, Binational Science Foundation, Fund for the Promotion of Research at the Technion, and Human Frontier Science Program to B.P. and by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health.
Abbreviations used:
- AJ
apical junction
- eff-1
epithelial fusion failure-1
- idf-1,
irregular dorsal fusion-1
- TEM
transmission electron microscopy.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0855) on January 17, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abmayr S. M., Balagopalan L., Galletta B. J., Hong S. J. Cell and molecular biology of myoblast fusion. Int. Rev. Cytol. 2003;225:33–89. doi: 10.1016/s0074-7696(05)25002-7. [DOI] [PubMed] [Google Scholar]
- Alper S., Kenyon C. REF-1, a protein with two bHLH domains, alters the pattern of cell fusion in C. elegans by regulating Hox protein activity. Development. 2001;128:1793–1804. doi: 10.1242/dev.128.10.1793. [DOI] [PubMed] [Google Scholar]
- Alper S., Kenyon C. The zinc finger protein REF-2 functions with the Hox genes to inhibit cell fusion in the ventral epidermis of C. elegans. Development. 2002;129:3335–3348. doi: 10.1242/dev.129.14.3335. [DOI] [PubMed] [Google Scholar]
- Anson M. Temperature dependence and Arrhenius activation energy of F-actin velocity generated in vitro by skeletal myosin. J. Mol. Biol. 1992;224:1029–1038. doi: 10.1016/0022-2836(92)90467-x. [DOI] [PubMed] [Google Scholar]
- Baird S. E., Fitch D. A., Kassem I.A.A., Emmons S. W. Pattern formation in the nematode epidermis: determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development. 1991;113:515–526. doi: 10.1242/dev.113.2.515. [DOI] [PubMed] [Google Scholar]
- Baron R., Neff L., Van P. T., Nefussi J., Vignery A. Kinetic and cytochemical identification of osteoclast precursors and their differentiation into multinucleated osteoclasts. Am. J. Pathol. 1986;122:363–378. [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R., Clague M. J., Durell S. R., Epand R. M. Membrane fusion. Chem. Rev. 2003;103:53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Bron R., Wahlberg J. M., Garoff H., Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993;12:693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassata G., Shemer G., Morandi P., Donhauser R., Podbilewicz B., Baumeister R. ceh-16/engrailed patterns the embryonic epidermis of Caenorhabditis elegans. Development. 2005;132:739–749. doi: 10.1242/dev.01638. [DOI] [PubMed] [Google Scholar]
- Ch'ng Q., Kenyon C. egl-27 generates anteroposterior patterns of cell fusion in C. elegans by regulating Hox gene expression and Hox protein function. Development. 1999;126:3303–3312. doi: 10.1242/dev.126.15.3303. [DOI] [PubMed] [Google Scholar]
- Chen E. H., Olson E. N. Unveiling the mechanisms of cell-cell fusion. Science. 2005;308:369–373. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- Chen E. H., Pryce B. A., Tzeng J. A., Gonzalez G. A., Olson E. N. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- Chen Z., Han M. C. elegans Rb, NuRD, and Ras regulate lin-39-mediated cell fusion during vulval fate specification. Curr. Biol. 2001;11:1874–1879. doi: 10.1016/s0960-9822(01)00596-6. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Frolov V. A., Leikina E., Bronk P., Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Kozlov M. M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Schoch C., Zech L., Blumenthal R. Gating kinetics of pH-activated membrane fusion of vesicular stomatitis virus with cells: stopped-flow measurements by dequenching of octadecylrhodamine fluorescence. Biochemistry. 1990;29:1303–1308. doi: 10.1021/bi00457a028. [DOI] [PubMed] [Google Scholar]
- Costa M., Raich W., Agbunag C., Leung B., Hardin J., Priess J. R. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli T., Pelletier S. L., Henis Y. I., White J. M. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J. Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo J. J., Opoku-Serebuoh E., Isaacson A. B., Scranton V. L., Tucker M., Han M., Mohler W. A. Fusogenic activity of EFF-1 is regulated via dynamic localization in fusing somatic cells of C. elegans. Curr. Biol. 2005;15:413–423. doi: 10.1016/j.cub.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Doberstein S. K., Fetter R. D., Mehta A. Y., Goodman C. S. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen R. B., Dispersyn G. D., Verheyen F. K., van den Eijnde S. M., Hofstra L., Thone F., Dijkstra P., Debie W., Borgers M., Ramaekers F. C. Partial cell fusion: a newly recognized type of communication between dedifferentiating cardiomyocytes and fibroblasts. Cardiovasc. Res. 2005;68:37–46. doi: 10.1016/j.cardiores.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Dutch R. E., Lamb R. A. Deletion of the cytoplasmic tail of the fusion protein of the paramyxovirus simian virus 5 affects fusion pore enlargement. J. Virol. 2001;75:5363–5369. doi: 10.1128/JVI.75.11.5363-5369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earles C. A., Bai J., Wang P., Chapman E. R. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 2001;154:1117–1123. doi: 10.1083/jcb.200105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber M. U., Ehler E., Billeter M. A., Naim H. Y. Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion. J. Virol. 2002;76:5720–5728. doi: 10.1128/JVI.76.11.5720-5728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C., Loren C. E., Grabbe C., Varshney G. K., Deleuil F., Hallberg B., Palmer R. H. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425:512–516. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- Firsching R., Buchholz C. J., Schneider U., Cattaneo R., ter Meulen V., Schneider-Schaulies J. Measles virus spread by cell-cell contacts: uncoupling of contact-mediated receptor (CD46) downregulation from virus uptake. J. Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. R., Waterston R. H. Muscle cell attachment in Caenorhabditis elegans. J. Cell Biol. 1991;114:465–479. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Marsh M., Gunther S., Pelchen-Matthews A., Stephens P., Ortlepp S., Stegmann T. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J. Virol. 1995;69:1462–1472. doi: 10.1128/jvi.69.3.1462-1472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie A. E., Brizzio V., Rose M. D. Distinct morphological phenotypes of cell fusion mutants. Mol. Biol. Cell. 1998;9:1395–1410. doi: 10.1091/mbc.9.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattegno T. Haifa: Technion-Israel Institute of Technology; 2003. Isolation and characterization of cell fusion mutants in C. elegans. Ph.D. thesis. [Google Scholar]
- Gerna G., Percivalle E., Baldanti F., Sozzani S., Lanzarini P., Genini E., Lilleri D., Revello M. G. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J. Virol. 2000;74:5629–5638. doi: 10.1128/jvi.74.12.5629-5638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons D., Erk I., Reilly B., Navaza J., Kielian M., Rey F., Lepault J. Visualization of the target-membrane-inserted fusion protein of Semliki Forest Virus by combined electron microscopy and crystallography. Cell. 2003;114:573–583. doi: 10.1016/s0092-8674(03)00683-4. [DOI] [PubMed] [Google Scholar]
- Gibbons D. L., Vaney M. C., Roussel A., Vigouroux A., Reilly B., Lepault J., Kielian M., Rey F. A. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature. 2004;427:320–325. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]
- Haller T., Dietl P., Pfaller K., Frick M., Mair N., Paulmichl M., Hess M. W., Furst J., Maly K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001;155:279–289. doi: 10.1083/jcb.200102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M. G., Walter P. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 2000;151:719–730. doi: 10.1083/jcb.151.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hird S. N., White J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 1993;121:1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., Martin I., Kahya N., Ruysschaert J. M., Pecheur E. On the mechanism of protein-induced membrane fusion: from model to biological membranes. Cell. Mol. Biol. Lett. 2002;7:231–232. [PubMed] [Google Scholar]
- Hu C., Ahmed M., Melia T. J., Sollner T. H., Mayer T., Rothman J. E. Fusion of cells by flipped SNAREs. Science. 2003;300:1745–1749. doi: 10.1126/science.1084909. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J. Biol. Chem. 1983;258:9108–9115. [PubMed] [Google Scholar]
- Jahn R., Lang T., Sudhof T. C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jaiswal J. K., Chakrabarti S., Andrews N. W., Simon S. M. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:E233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon N., Gilula N. B. Membrane events involved in myoblast fusion. J. Cell Biol. 1979;81:411–425. doi: 10.1083/jcb.81.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D., Zimmerberg J., Puri A., Sarkar D. P., Blumenthal R. Single cell fusion events induced by influenza hemagglutinin: studies with rapid-flow, quantitative fluorescence microscopy. Exp. Cell Res. 1991;195:137–144. doi: 10.1016/0014-4827(91)90509-s. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A gene involved in the development of the posterior body region of C. elegans. Cell. 1986;46:477–487. doi: 10.1016/0092-8674(86)90668-9. [DOI] [PubMed] [Google Scholar]
- Knust E., Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Koh K., Peyrot S. M., Wood C. G., Wagmaister J. A., Maduro M. F., Eisenmann D. M., Rothman J. H. Cell fates and fusion in the C. elegans vulval primordium are regulated by the EGL-18 and the ELT-6 GATA factors—apparent direct targets of the LIN-39 Hox protein. Development. 2002;129:5171–5180. doi: 10.1242/dev.129.22.5171. [DOI] [PubMed] [Google Scholar]
- Koh K., Rothman J. H. ELT-5 and ELT-6 are required continuously to regulate epidermal seam cell differentiation and cell fusion in C. elegans. Development. 2001;128:2867–2880. doi: 10.1242/dev.128.15.2867. [DOI] [PubMed] [Google Scholar]
- Kontani K., Moskowitz I. P., Rothman J. H. Repression of cell-cell fusion by components of the C. elegans vacuolar ATPase complex. Dev. Cell. 2005;8:787–794. doi: 10.1016/j.devcel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Koppen M., Simske J. S., Sims P. A., Firestein B. L., Hall D. H., Radice A. D., Rongo C., Hardin J. D. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 2001;3:983–991. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- Kozlov M. M., Chernomordik L. V. The protein coat in membrane fusion: lessons from fission. Traffic. 2002;3:256–267. doi: 10.1034/j.1600-0854.2002.030403.x. [DOI] [PubMed] [Google Scholar]
- Lee J., Lentz B. R. Secretory and viral fusion may share mechanistic events with fusion between curved lipid bilayers. Proc. Natl. Acad. Sci. USA. 1998;95:9274–9279. doi: 10.1073/pnas.95.16.9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E., Mittal A., Cho M. S., Melikov K., Kozlov M. M., Chernomordik L. V. Influenza hemagglutinins outside of the contact zone are necessary for fusion pore expansion. J. Biol. Chem. 2004;279:26526–26532. doi: 10.1074/jbc.M401883200. [DOI] [PubMed] [Google Scholar]
- McInerney G. M., Smit J. M., Liljestrom P., Wilschut J. Semliki Forest virus produced in the absence of the 6K protein has an altered spike structure as revealed by decreased membrane fusion capacity. Virology. 2004;325:200–206. doi: 10.1016/j.virol.2004.04.043. [DOI] [PubMed] [Google Scholar]
- McKeown C., Praitis V., Austin J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development. 1998;125:2087–2098. doi: 10.1242/dev.125.11.2087. [DOI] [PubMed] [Google Scholar]
- Melikyan G. B., Markosyan R. M., Hemmati H., Delmedico M. K., Lambert D. M., Cohen F. S. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 2000;151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Mittal A., Leikina E., Chernomordik L. V., Bentz J. Kinetically differentiating influenza hemagglutinin fusion and hemifusion machines. Biophys. J. 2003;85:1713–1724. doi: 10.1016/S0006-3495(03)74601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler W. A., Shemer G., del Campo J., Valansi C., Opoku-Serebuoh E., Scranton V., Assaf N., White J. G., Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion in C. elegans. Dev. Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Mohler W. A., Simske J. S., Williams-Masson E. M., Hardin J. D., White J. G. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr. Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Munoz-Barroso I., Durell S., Sakaguchi K., Appella E., Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C. Q., Hall D. H., Yang Y., Fitch D.H.A. Morphogenesis of the Caenorhabditis elegans male tail tip. Dev. Biol. 1999;207:86–106. doi: 10.1006/dbio.1998.9173. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Li X., Tiensuu T., Auty R., Greenwald I., Tuck S. Caenorhabditis elegans lin-25: cellular focus, protein expression and requirement for sur-2 during induction of vulval fates. Development. 1998;125:4809–4819. doi: 10.1242/dev.125.23.4809. [DOI] [PubMed] [Google Scholar]
- Nolan S., Cowan A. E., Koppel D. E., Jin H., Grote E. FUS1 regulates the opening and expansion of fusion pores between mating yeast. Mol. Biol. Cell. 2006;17:2439–2450. doi: 10.1091/mbc.E05-11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A. F., Monck J. R., Fernandez J. M. Events leading to the opening and closing of the exocytotic fusion pore have markedly different temperature dependencies. Biophys. J. 1992;61:800–809. doi: 10.1016/S0006-3495(92)81884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F., Weber T., McNew J. A., Westermann B., Sollner T. H., Rothman J. E. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen T., Kielian M. Cholesterol is required for infection by semliki forest virus. J. Cell Biol. 1991;112:615–623. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsky I., Zimmerberg J. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J. Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbilewicz B. Membrane fusion as a morphogenetic force in nematode development. Nematology. 2000;2:99–111. [Google Scholar]
- Podbilewicz B. WormBook, editor. Cell fusion. The C. elegans Research Community: WormBook, doi/10.1895/wormbook.1.52.1. 2006 doi: 10.1895/wormbook.1.52.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Podbilewicz B., Chernomordik L. V. Cell fusion in development and disease. In: Tamm L. K., editor. Protein-Lipids Interactions. New York: Wiley-VCH; 2005. pp. 221–244. [Google Scholar]
- Podbilewicz B., Leikina E., Sapir A., Valansi C., Suissa M., Shemer G., Chernomordik L. V. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev. Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B., White J. G. Cell fusions in the developing epithelia of C. elegans. Dev. Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Priess J. R., Hirsh D. I. Caenorhabditis elegans morphogenesis: The role of cytoskeleton in elongation of the embryo. Dev. Biol. 1986;117:156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- Rabin Y., Podbilewicz B. Temperature-controlled microscopy for imaging living cells: apparatus, thermal analysis, and temperature dependency of embryonic elongation in C. elegans. J. Microsc. 2000;199(Pt 3):214–223. doi: 10.1046/j.1365-2818.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- Rushton E., Drysdale R., Abmayr S. M., Michelson A. M., Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- Scepek S., Coorssen J. R., Lindau M. Fusion pore expansion in horse eosinophils is modulated by Ca2+ and protein kinase C via distinct mechanisms. EMBO J. 1998;17:4340–4345. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C., Blumenthal R., Clague M. J. A long-lived state for influenza virus-erythrocyte complexes committed to fusion at neutral pH. FEBS Lett. 1992;311:221–225. doi: 10.1016/0014-5793(92)81107-w. [DOI] [PubMed] [Google Scholar]
- Sharma-Kishore R., White J. G., Southgate E., Podbilewicz B. Formation of the vulva in C. elegans: a paradigm for organogenesis. Development. 1999;126:691–699. doi: 10.1242/dev.126.4.691. [DOI] [PubMed] [Google Scholar]
- Shemer G. Haifa: Technion-Israel Institute for Technology; 2002. Cell fusion and organogenesis in Caenorhabditis elegans. Ph.D. thesis. [Google Scholar]
- Shemer G., Kishore R., Podbilewicz B. Ring formation drives invagination of the vulva in C. elegans: Ras, cell fusion and cell migration determine structural fates. Dev. Biol. 2000;221:233–248. doi: 10.1006/dbio.2000.9657. [DOI] [PubMed] [Google Scholar]
- Shemer G., Podbilewicz B. LIN-39/Hox triggers cell division and represses EFF-1/Fusogen-dependent vulval cell fusion. Genes Dev. 2002;16:3136–3141. doi: 10.1101/gad.251202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer G., Podbilewicz B. The story of cell fusion: big lessons from little worms. BioEssays. 2003;25:672–682. doi: 10.1002/bies.10301. [DOI] [PubMed] [Google Scholar]
- Shemer G., Suissa M., Kolotuev I., Nguyen K.C.Q., Hall D. H., Podbilewicz B. EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans. Curr. Biol. 2004;14:1587–1591. doi: 10.1016/j.cub.2004.07.059. [DOI] [PubMed] [Google Scholar]
- Stegmann T., White J. M., Helenius A. Intermediates in Influenza induced membrane fusion. EMBO J. 1990;9:4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Jovine L., Litscher E. S. A profile of fertilization in mammals. Nat. Cell Biol. 2001;3:E59–E64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- Witze E., Rothman J. H. Cell Fusion: an EFFicient sculptor. Curr. Biol. 2002;12:R467–R469. doi: 10.1016/s0960-9822(02)00948-x. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang Y., Fitch D. A., Herman M. E. TLP-1 is an asymmetric cell fate determinant that responds to Wnt signals and controls male tail tip morphogenesis. Development. 2002;129:1497–1508. doi: 10.1242/dev.129.6.1497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.