Abstract
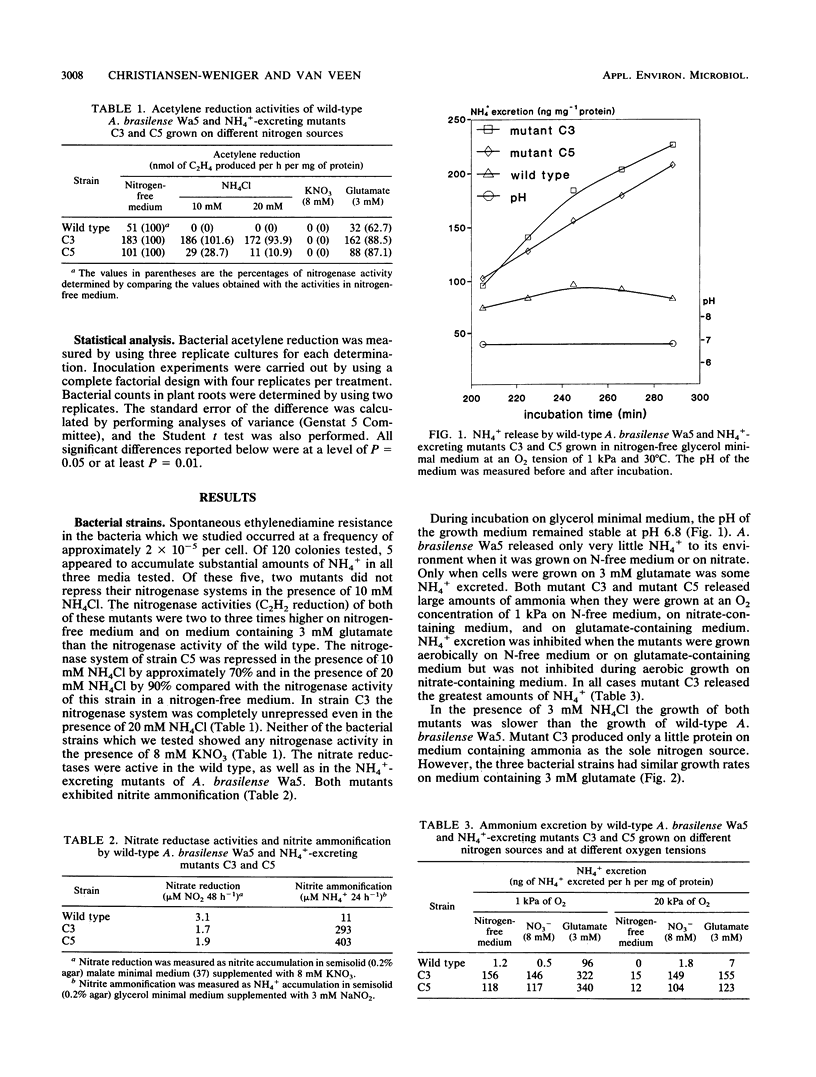
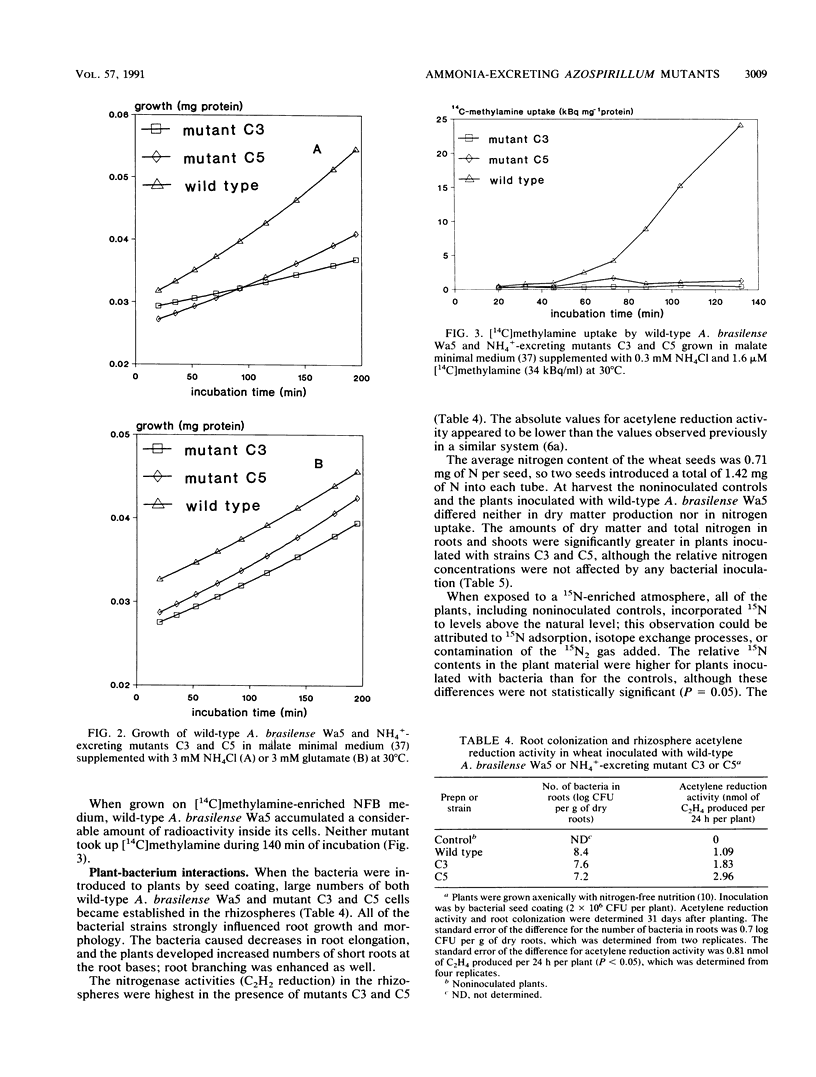
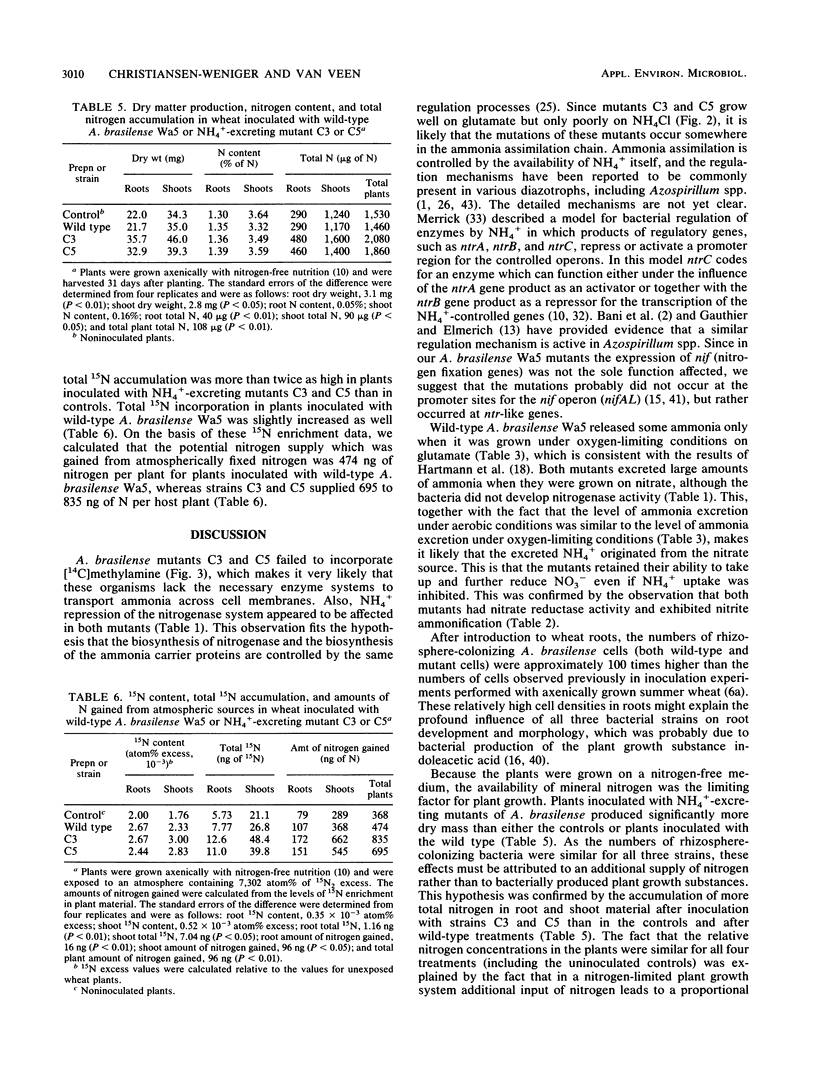
Spontaneous ethylenediamine-resistant mutants of Azospirillum brasilense were selected on the basis of their excretion of NH4+. Two mutants exhibited no repression of their nitrogenase enzyme systems in the presence of high (20 mM) concentrations of NH4+. The nitrogenase activities of these mutants on nitrogen-free minimal medium were two to three times higher than the nitrogenase activity of the wild type. The mutants excreted substantial amounts of ammonia when they were grown either under oxygen-limiting conditions (1 kPa of O2) or aerobically on nitrate or glutamate. The mutants grew well on glutamate as a sole nitrogen source but only poorly on NH4Cl. Both mutants failed to incorporate [14C]methylamine. We demonstrated that nitrite ammonification occurs in the mutants. Wild-type A. brasilense, as well as the mutants, became established in the rhizospheres of axenically grown wheat plants at levels of > 107 cells per g of root. The rhizosphere acetylene reduction activity was highest in the preparations containing the mutants. When plants were grown on a nitrogen-free nutritional medium, both mutants were responsible for significant increases in root and shoot dry matter compared with wild-type-treated plants or with noninoculated controls. Total plant nitrogen accumulation increased as well. When they were exposed to a 15N2-enriched atmosphere, both A. brasilense mutants incorporated significantly higher amounts of 15N inside root and shoot material than the wild type did. The results of our nitrogen balance and 15N enrichment studies indicated that NH4+-excreting A. brasilense strains potentially support the nitrogen supply of the host plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eskew D. L., Eaglesham A. R., App A. A. Heterotrophic n(2) fixation and distribution of newly fixed nitrogen in a rice-flooded soil system. Plant Physiol. 1981 Jul;68(1):48–52. doi: 10.1104/pp.68.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espin G., Alvarez-Morales A., Cannon F., Dixon R., Merrick M. Cloning of the glnA, ntrB and ntrC genes of Klebsiella pneumoniae and studies of their role in regulation of the nitrogen fixation (nif) gene cluster. Mol Gen Genet. 1982;186(4):518–524. doi: 10.1007/BF00337959. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Fischer M., Levy E., Geller T. Regulatory mutation that controls nif expression and histidine transport in Azospirillum brasilense. J Bacteriol. 1986 Jul;167(1):423–426. doi: 10.1128/jb.167.1.423-426.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Fu H. A., Burris R. H. Influence of amino acids on nitrogen fixation ability and growth of Azospirillum spp. Appl Environ Microbiol. 1988 Jan;54(1):87–93. doi: 10.1128/aem.54.1.87-93.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito O., Cabrera D., Watanabe I. Fixation of dinitrogen-15 associated with rice plants. Appl Environ Microbiol. 1980 Mar;39(3):554–558. doi: 10.1128/aem.39.3.554-558.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. Ammonium (methylammonium) transport by Klebsiella pneumoniae. Biochim Biophys Acta. 1982 Jun 28;688(3):702–708. doi: 10.1016/0005-2736(82)90282-6. [DOI] [PubMed] [Google Scholar]
- Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta. 1981 Nov 9;639(1):41–52. doi: 10.1016/0304-4173(81)90004-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J. A new model for nitrogen control. Nature. 1982 Jun 3;297(5865):362–363. doi: 10.1038/297362a0. [DOI] [PubMed] [Google Scholar]
- Neyra C. A., Van Berkum P. Nitrate reduction nitrogenase activity in Spirillum lipoferum1. Can J Microbiol. 1977 Mar;23(3):306–310. doi: 10.1139/m77-045. [DOI] [PubMed] [Google Scholar]
- Nur I., Okon Y., Henis Y. An increase in nitrogen content of Setaria italica and Zea mays inoculated with Azospirillum. Can J Microbiol. 1980 Apr;26(4):482–485. doi: 10.1139/m80-080. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Methods for Growing Spirillum lipoferum and for Counting It in Pure Culture and in Association with Plants. Appl Environ Microbiol. 1977 Jan;33(1):85–88. doi: 10.1128/aem.33.1.85-88.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Heytler P. G., Hardy R. W. N(2) Fixation by Azospirillum brasilense and Its Incorporation into Host Setaria italica. Appl Environ Microbiol. 1983 Sep;46(3):694–697. doi: 10.1128/aem.46.3.694-697.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. Use of 15N-enriched soil to study N2 fixation in grasses. Basic Life Sci. 1977;9:473–482. [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


