Abstract
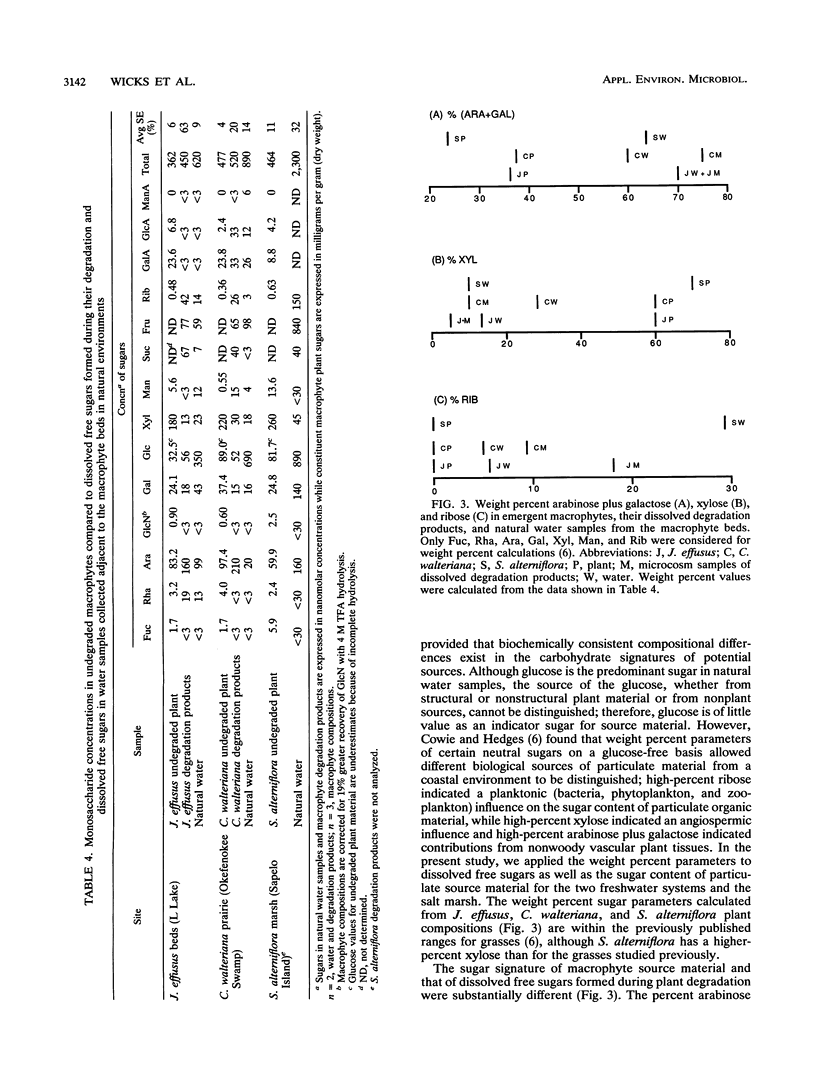
The sugar contents of emergent macrophytes from a freshwater lake, a freshwater swamp, and a salt marsh in the southeastern United States were examined together with the dissolved free sugars produced during macrophyte degradation and in natural water samples collected adjacent to macrophyte stands. Simultaneous separation of up to 13 neutral and 2 amino sugars together with 3 uronic acids and muramic acid was achieved by anion-exchange high-performance ion chromatography. As little as 10 pmol or a concentration of 20 nM sugar can be detected by pulsed amperometry, a greater sensitivity for sugar quantification than that of previously reported detection techniques used in conjunction with either gas or liquid chromatographic systems. Optimum conditions for hydrolysis of plant material by using trifluoroacetic acid were determined, and internal standards were used to quantify losses due to matrix effects and solid-phase extraction of samples. Our data demonstrate that ratios of certain indicator sugars in undegraded macrophytes differ significantly from ratios of dissolved free sugars formed during macrophyte degradation, reflecting the complex processes (biological and physical) involved in vascular plant degradation in aquatic ecosystems. Natural water samples collected adjacent to macrophyte beds contained dissolved free sugars at concentrations of 620 nM (lake), 890 nM (freshwater swamp), and 2,300 nM (salt marsh). Sugar signatures of these natural water samples were similar to those of macrophyte degradation products.
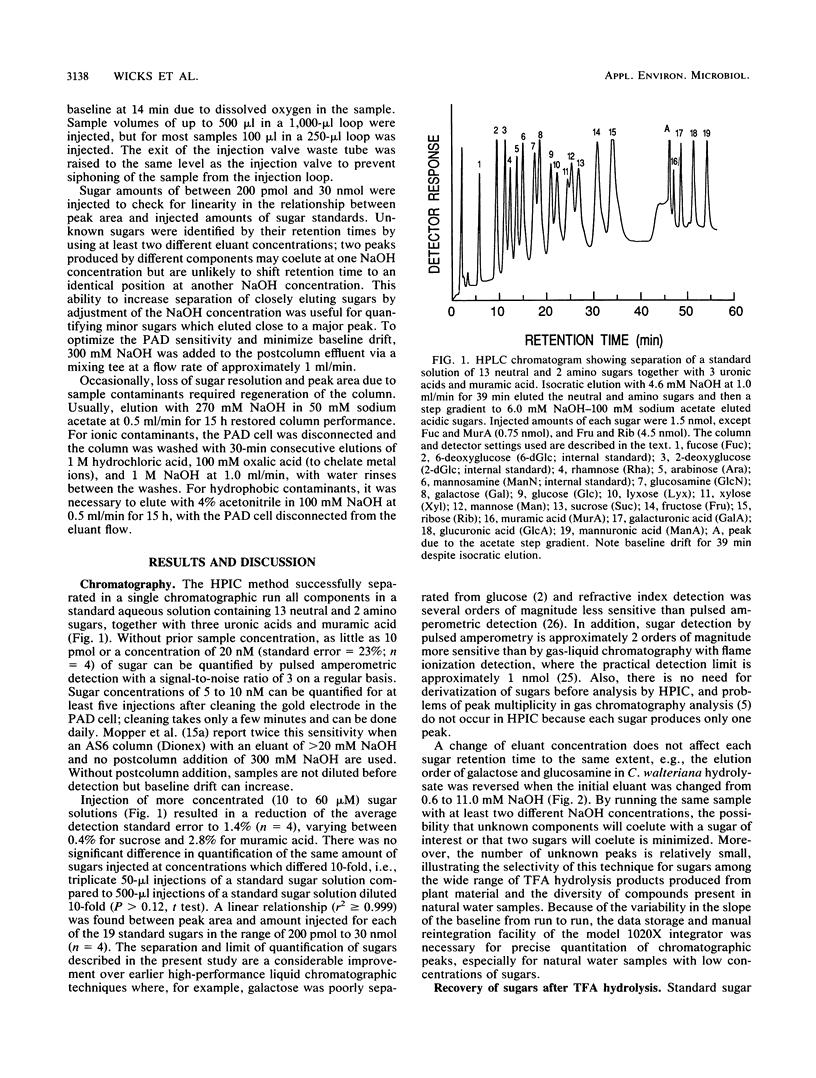
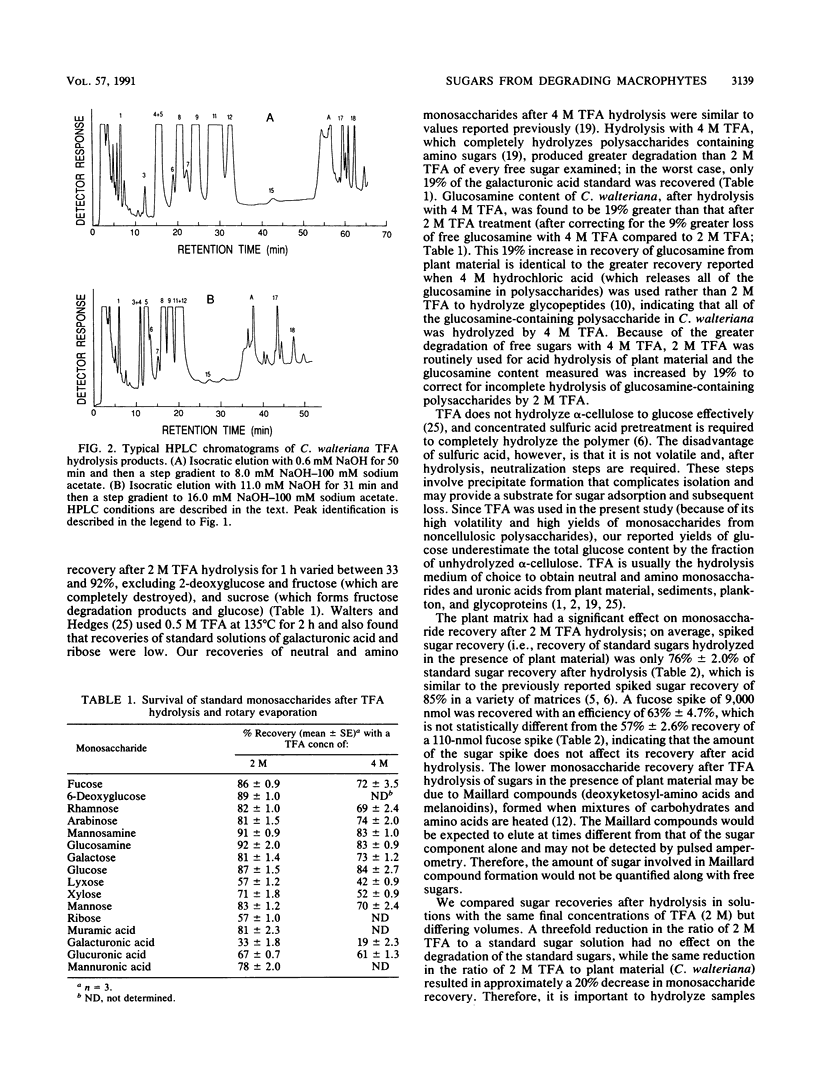
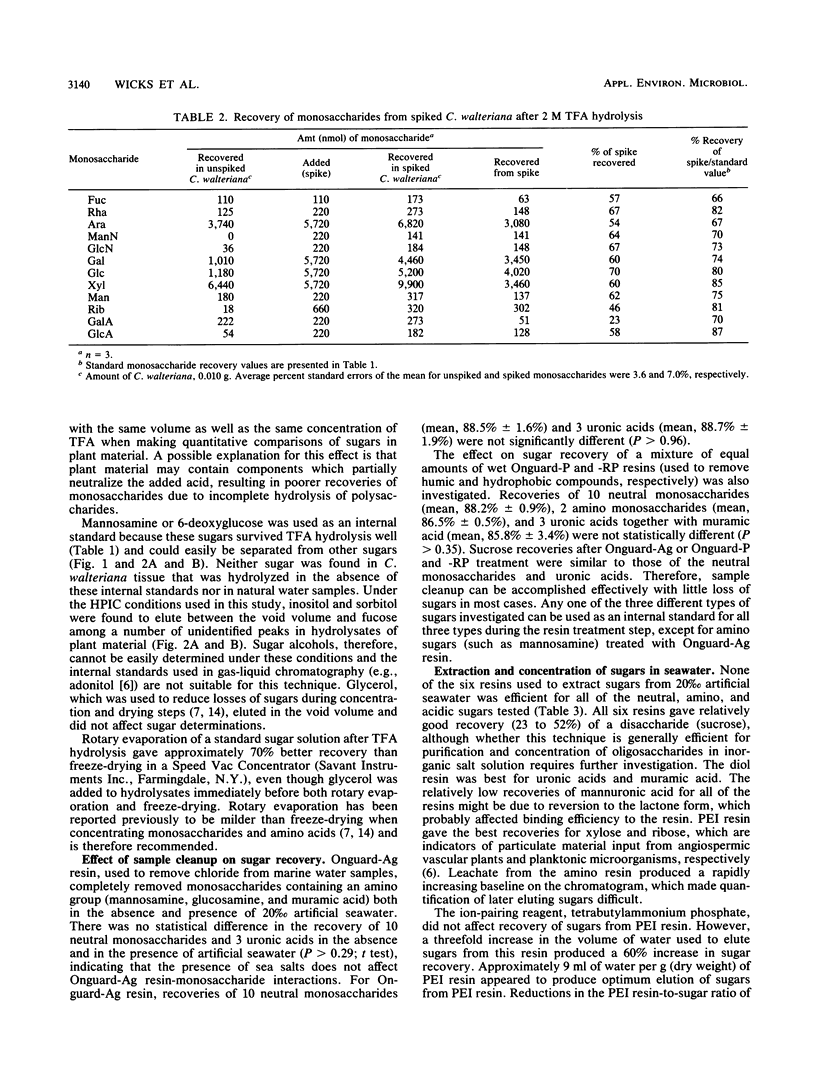
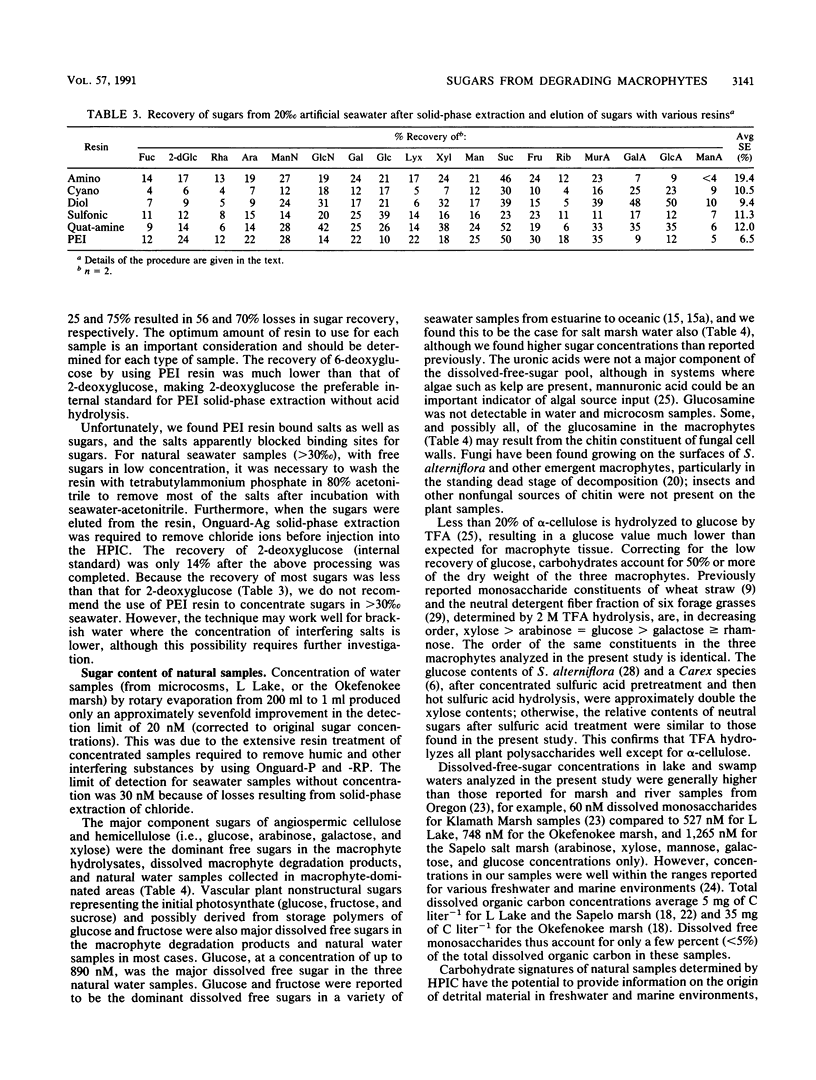
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson R., Mopper K. A note on the losses of monosaccharides, amino sugars, and amino acids from extracts during concentration procedures. Anal Biochem. 1978 Jan;84(1):186–190. doi: 10.1016/0003-2697(78)90498-0. [DOI] [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Lee Y. C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988 Apr;170(1):54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Hurrell R. F., Carpenter K. J. Mechanisms of heat damage in proteins. 8. The role of sucrose in the susceptibility of protein foods to heat damage. Br J Nutr. 1977 Sep;38(2):285–297. doi: 10.1079/bjn19770089. [DOI] [PubMed] [Google Scholar]
- Moran M. A., Hodson R. E. Bacterial secondary production on vascular plant detritus: relationships to detritus composition and degradation rate. Appl Environ Microbiol. 1989 Sep;55(9):2178–2189. doi: 10.1128/aem.55.9.2178-2189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Schweizer T. F. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugars (as O-methyloxime acetates), and a study on hydrolytic conditions for glycoproteins and polysaccharides in order to increase sugar recoveries. Anal Biochem. 1984 Oct;142(1):58–67. doi: 10.1016/0003-2697(84)90516-5. [DOI] [PubMed] [Google Scholar]


