Abstract
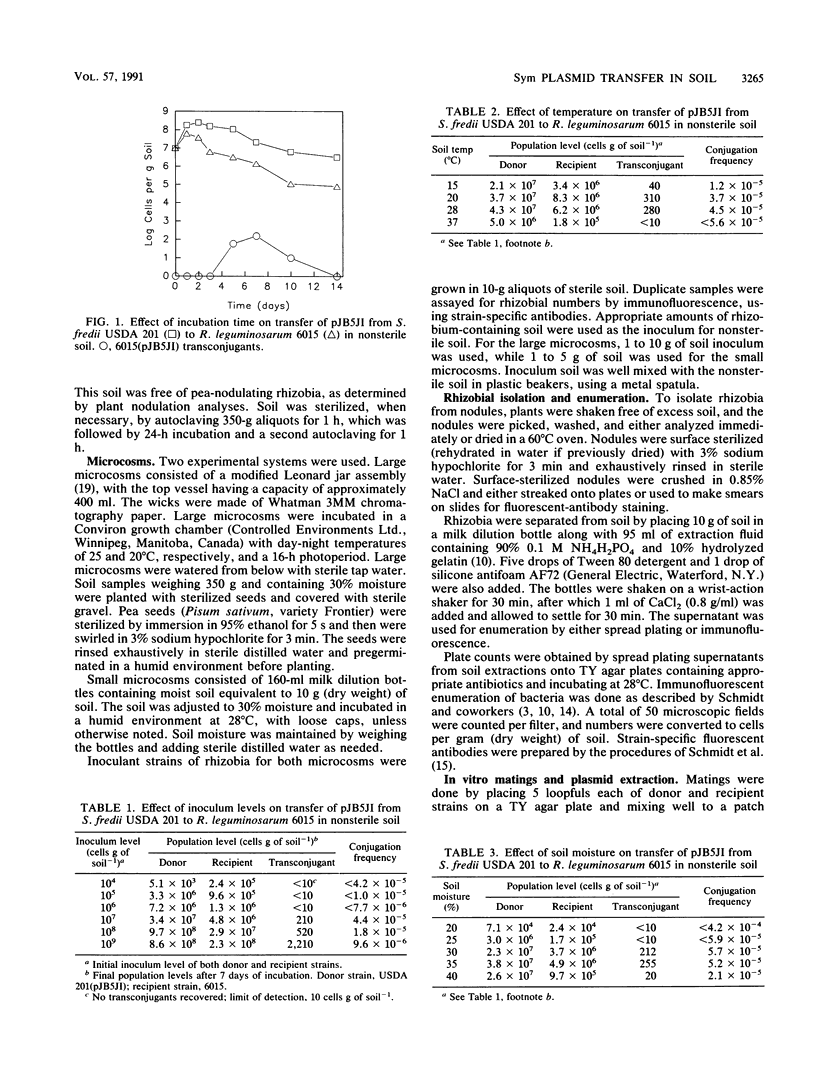
Transfer of the pea (Pisum sativum L.) symbiotic plasmid pJB5JI between strains of rhizobia was examined in sterile and nonsterile silt loam soil. Sinorhizobium fredii USDA 201 and HH003 were used as plasmid donors, and symbiotic plasmid-cured Rhizobium leguminosarum 6015 was used as the recipient. The plasmid was carried but not expressed in S. fredii strains, whereas transfer of the plasmid to R. leguminosarum 6015 rendered the recipient capable of nodulating pea plants. Confirmation of plasmid transfer was obtained by acquisition of plasmid-encoded antibiotic resistance genes, nodulation of pea plants, and plasmid profiles. Plasmid transfer in nonsterile soil occurred at frequencies of up to 10−4 per recipient and appeared to be highest at soil temperatures and soil moisture levels optimal for rhizobial growth. Conjugation frequencies were usually higher in sterile soil than in nonsterile soil. In nonsterile soil, transconjugants were recovered only with strain USDA 201 as the plasmid donor. Increasing the inoculum levels of donor and recipient strains up to 109 cells g of soil−1 increased the number of transconjugants; peak plasmid transfer frequencies, however, were found at the lower inoculum level of 107 cells g of soil−1. Plasmid transfer frequencies were raised in the presence of the pea rhizosphere or by additions of plant material. Transconjugants formed by the USDA 201(pJB5JI) × 6015 mating in soil formed effective nodules on peas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale M. J., Fry J. C., Day M. J. Transfer and occurrence of large mercury resistance plasmids in river epilithon. Appl Environ Microbiol. 1988 Apr;54(4):972–978. doi: 10.1128/aem.54.4.972-978.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Heron D. S., Pueppke S. G. Mode of infection, nodulation specificity, and indigenous plasmids of 11 fast-growing Rhizobium japonicum strains. J Bacteriol. 1984 Dec;160(3):1061–1066. doi: 10.1128/jb.160.3.1061-1066.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Levin B. R., Stewart F. M., Rice V. A. The kinetics of conjugative plasmid transmission: fit of a simple mass action model. Plasmid. 1979 Apr;2(2):247–260. doi: 10.1016/0147-619x(79)90043-x. [DOI] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius-Güth Inge-M, Pühler Alfred, Simon Reinhard. Conjugal Transfer of Megaplasmid 2 between Rhizobium meliloti Strains in Alfalfa Nodules. Appl Environ Microbiol. 1990 Aug;56(8):2354–2359. doi: 10.1128/aem.56.8.2354-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaume A., Angle J. S., Sadowsky M. J. Influence of soil variables on in situ plasmid transfer from Escherichia coli to Rhizobium fredii. Appl Environ Microbiol. 1989 Jul;55(7):1730–1734. doi: 10.1128/aem.55.7.1730-1734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Gibson A. H., Dudman W. F., Watson J. M. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl Environ Microbiol. 1987 Dec;53(12):2942–2947. doi: 10.1128/aem.53.12.2942-2947.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L. Dynamics of plasmid transfer on surfaces. J Gen Microbiol. 1990 Jun;136(6):1001–1007. doi: 10.1099/00221287-136-6-1001. [DOI] [PubMed] [Google Scholar]
- Wellington E. M., Cresswell N., Saunders V. A. Growth and survival of streptomycete inoculants and extent of plasmid transfer in sterile and nonsterile soil. Appl Environ Microbiol. 1990 May;56(5):1413–1419. doi: 10.1128/aem.56.5.1413-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]