Abstract
Adeno-associated virus (AAV) is a nonpathogenic parvovirus that efficiently replicates in the presence of adenovirus (Ad). Exogenous expression of the AAV replication proteins induces caspase-dependent apoptosis, but determining if AAV infection causes apoptosis during viral infection is complicated by Ad-mediated programmed cell death. To eliminate Ad-induced cytolysis, we used an E3 Adenoviral Death Protein (ADP) mutant, pm534. AAV and pm534-coinfected cells exhibited increased cell killing compared to pm534 alone. Relative to cells infected with Ad alone, AAV and wild type Ad-infected cells displayed decreased ADP expression, increased cytolysis until the third day of the infection, and decreased cytolysis thereafter. Biochemical and morphological characteristics of apoptosis were observed during coinfections with AAV and pm534 or Ad, including a moderate degree of caspase activation that was not present during infections with pm534 or Ad alone. AAV coinfection also increased extracellular pH. These studies suggest that AAV induces caspase-dependent and caspase-independent apoptosis.
INTRODUCTION
Adenovirus (Ad) is a non-enveloped, icosahedral virus that replicates in the nucleus. During lytic infections, temporal regulation of cell death is an important aspect of Ad propagation. If cell death were induced prior to the assembly and encapsidation of progeny virions, there would be a severe decline in Ad production. However, once the viral replicative cycle is complete, cytolysis favors viral release and secondary infections. Several studies indicate that the E3-11.6K adenoviral death protein (ADP) is responsible for late phase cell killing (Tollefson et al., 1996a; Tollefson et al., 1996b). Unlike the other E3 proteins, ADP is only expressed in small amounts during the early phase of Ad infection. This is followed by a 400-fold increase in its synthesis from the major late promoter by 28 to 30 hours post-infection (pi) (Tollefson et al., 1992). This temporal regulation allows ADP to induce cell death exclusively during the late stages of Ad infection. The effects of ADP on cell viability are dramatic. While cells infected with wild-type Ad begin to lyse 2 to 3 days pi, cells infected with an ADP mutant do not lyse until 6 or 7 days pi (Tollefson et al., 1996a; Tollefson et al., 1996b). Additionally, infection with ADP mutants results in smaller plaques that are slower to develop than those induced by wild-type Ad infection (Tollefson et al., 1996a; Tollefson et al., 1996b). These studies illustrate the pivotal role that ADP expression plays in Ad-induced cytolysis and viral spread.
Previous studies indicate that ADP-induced cell death is a form of non-apoptotic programmed cell death (Kanj et al., 2006; Zou et al., 2004). While necrosis is an uncontrolled destruction of cell structure in which the cells swell and burst, apoptosis is a regulated means of inducing cell death that is characterized by cell shrinkage, membrane blebbing, chromatin condensation, DNA fragmentation, and caspase activation. Although the term apoptosis is often used synonymously with programmed cell death, there are an increasing number of studies describing non-apoptotic, caspase-independent programmed cell death (Hetz, Torres, and Quest, 2005). Recent studies suggest that ADP overexpression from the Ad major late promoter induces caspase-dependent apoptosis. However, in the context of a wild type Ad infection, ADP induces non-apoptotic cell death that lacks the biochemical and morphological characteristics of caspase-dependent apoptosis (Kanj et al., 2006; Zou et al., 2004).
Adenovirus is the most efficient helper virus for the nonpathogenic parvovirus adeno-associated virus (AAV). Although AAV requires the presence of a helper virus for a fully permissive infection, AAV inhibits Ad replication and gene expression during coinfection (Casto et al., 1967; Casto, Atchison, and Hammon, 1967). AAV also acts as a tumor suppressor, restricts Ad-induced cellular proliferation (Khleif et al., 1991; Ostrove, Duckworth, and Berns, 1981), and sensitizes cells to chemotherapeutic agents (Schlehofer, 1994). The AAV genome contains two open reading frames, rep and cap, which encode the nonstructural replication proteins (Rep) and structural capsid proteins (Cap) respectively. Previous studies indicate that expression of AAV Rep proteins is sufficient to induce apoptosis. In an inducible cell line, Rep expression combined with UV irradiation resulted in chromatin condensation, DNA fragmentation, poly-ADP ribose polymerase (PARP) cleavage, and rapid cell death in which the plasma membranes primarily remained intact (Zhou and Trempe, 1999; Zhou, Yang, and Trempe, 1999). This suggests that Rep expression induces caspase-activation and apoptosis in damaged cells. In another study, Rep78 expression following plasmid transfection disrupted the cell cycle, induced caspase activation, and initiated p53-independent apoptosis (Schmidt, Afione, and Kotin, 2000). Although these studies were not conducted in the context of a coinfection, they indicate that AAV infection may induce apoptosis as a result of Rep expression. The effects of AAV infection on programmed cell death have not been determined to date, primarily because AAV's reliance on a helper virus has made it difficult to separate the effects of AAV on apoptosis from that of Ad. To eliminate Ad-induced cell killing during the first five to six days of infection, we used an ADP mutant as a helper virus. In doing so, we were able to obtain novel insights into the effects of AAV on cell viability and apoptosis. Productive AAV infection induced biochemical and morphological characteristics of apoptosis, but only moderate caspase involvement was observed. These studies suggest that AAV induces both caspase-dependent and caspase-independent apoptosis, thereby providing the first insight into AAV-mediated apoptosis during coinfection.
RESULTS
AAV decreases ADP protein levels
We have previously reported that the smallest Ad E3 transcript, which encodes ADP, was virtually undetectable by Northern analysis following coinfection with AAV (Timpe, Verrill, and Trempe, 2006). Since ADP plays a central role in cytolysis during the late phase of Ad infection, we decided to examine the effects of decreased ADP expression on cell viability and apoptosis. Throughout this study, we utilized pm534, an Ad5-derived mutant that lacks ADP expression. We first confirmed that ADP protein levels decreased during AAV coinfections. Monolayers of A549 cells were infected with Ad5 or pm534 in the presence or absence of AAV. Cells were harvested two days post-infection (pi) and assayed by immunoblot. As shown in Figure 1, ADP protein was expressed in cells infected with wild-type Ad5 but not in cells infected with the ADP mutant pm534. The addition of 100 IU of AAV strongly reduced, but did not eliminate, ADP expression in Ad5-infected cells. This demonstrates that AAV inhibits ADP expression during coinfection. During the early phase of infections, Ad expresses considerable amounts of E2A protein, which is required for Ad DNA replication. We therefore used an E2A-specific antibody to confirm early adenovirus protein expression. Abundant E2A protein was observed during infections with both strains of adenovirus. The slight reduction in pm534-infected cells relative to wild type Ad5 may indicate that the ADP mutant may establish a less robust infection. In the Rep panel, the presence of AAV proteins at both assayed time points suggests that Ad5 and pm534 supported AAV gene expression, although slightly lower Rep levels were present in pm534-infected cells. Rep-associated activity is confirmed in the AAV-infected lanes of the E2A panel since previous studies have demonstrated that AAV Rep proteins inhibit E2A expression (Jing et al., 2001; Nada and Trempe, 2002; Timpe, Verrill, and Trempe, 2006). Thus, decreased E2A in AAV-coinfected samples relative to Ad5 or pm534 alone is indicative of AAV-mediated inhibition of gene expression. Together, these data suggest that 100 IU of AAV is sufficient to substantially reduce ADP protein expression during coinfection, that pm534 and Ad5 establish productive infections, and that both Ad viruses provide the necessary helper functions for a permissive AAV infection.
Figure 1.
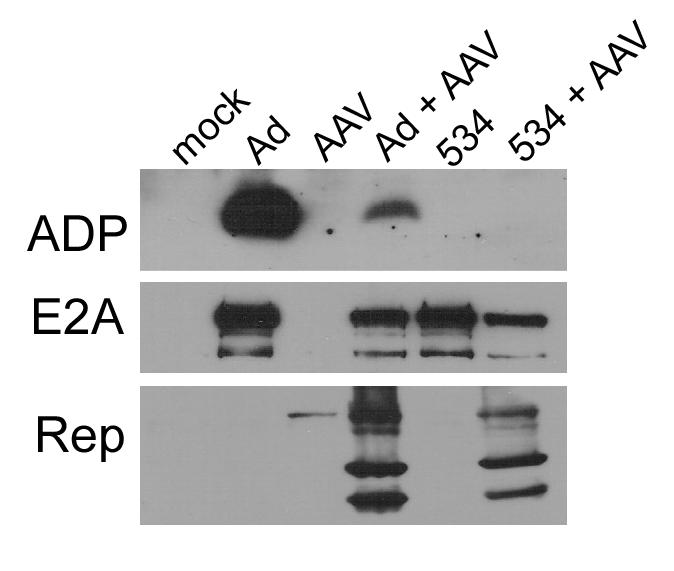
Western analysis of Ad and AAV proteins during coinfections. A549 cells were infected with Ad5 (10 moi), pm534 (10 moi), and/or AAV (100 IU) as indicated at the top of the figure. Cultures were harvested 48h pi. Equal amounts of protein were separated by SDS-PAGE and probed with the antibody denoted on the left of the images.
AAV alters cell lysis during coinfection
Since ADP induces cell death during Ad infection, it stands to reason that AAV-mediated inhibition of ADP expression could decrease cell death, thereby prolonging the lifespan of coinfected cells. To test the effects of AAV and ADP expression on cell viability, we utilized the lactate dehydrogenase (LDH) assay in which NADH oxidation is directly proportional to extracellular LDH concentration and cell lysis. AAV-infected or naïve A549 cells were infected with Ad5 or pm534. Aliquots of media were removed 24 h pi, and daily thereafter from each well and assayed for LDH activity. Low levels of NADH oxidation, which correspond to low released LDH activity, indicate that little cell lysis has occurred. As shown in Fig, 2A, the majority of uninfected cells remained intact throughout the assay. Because AAV is a defective virus and is unable to initiate a productive infection in the absence of a helper virus, AAV infection mimicked uninfected cells. In contrast, infection with wild-type Ad5 induced rapid cytolysis between days 3 and 4. Loss of ADP abrogates this effect, thereby allowing pm534-infected cells to remain intact for an extended period of time. As a result, cells infected with pm534 exhibited the same pattern of cell lysis as uninfected cells. It was interesting that AAV induced cell lysis during pm534 coinfection. Since pm534 does not express ADP, this cell killing was introduced by an alternative mechanism than wild-type Ad-induced programmed cell death. Surprisingly, cells coinfected with Ad5 and AAV exhibited a markedly different cell lysis profile than either Ad5 or pm534. Relative to Ad5 alone, AAV coinfection increased cytolysis during the first three days of Ad5 infection, but decreased cell lysis thereafter. Also, there was a steady increase in lysis of Ad5/AAV coinfected cells throughout the course of the assay, in contrast with the steep slope of Ad5-induced cell death between 3 and 4 days pi. Rapid induction of cell death during Ad5 infection was due to ADP activity, which causes cells to lyse when they are full of progeny virions. Inhibition of ADP expression during AAV/Ad5 coinfection is probably responsible for the decrease in cell killing after 3 days pi, but it does not explain the early increase in cell killing. Since previous reports demonstrated that AAV Rep protein expression induces apoptosis in the absence of adenovirus, we hypothesized that productive AAV infection mediates ADP-independent cell killing by inducing apoptosis. This hypothesis is supported by the induction of cytolysis during pm534/AAV coinfections. Since pm534 alone did not induce cell killing, the cytolysis observed during pm534/AAV coinfections can be attributed to the effects of AAV. However, cell lysis occurred later in the pm534/AAV infections than Ad5/AAV infections. This could be due to the slight reduction in Rep expression during pm534 coinfection relative to Ad5 infection (Figure 1). In addition, ADP is reduced but not eliminated during coinfection with AAV, so the early onset of cytolysis in AAV/Ad5 coinfected cells relative to AAV/pm534 infected cells could reflect both ADP- and AAV-induced cell lysis.
Figure 2.
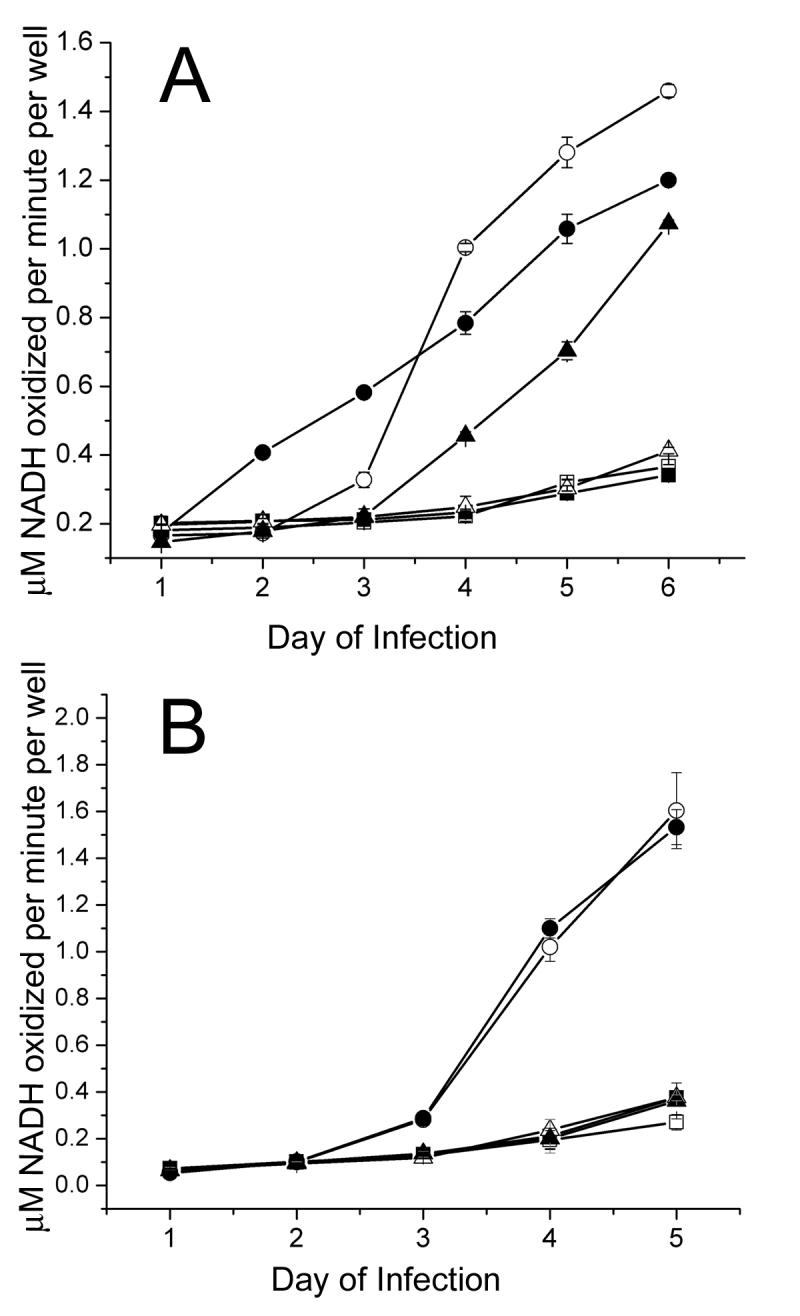
AAV altered lysis of Ad infected cells. A549 cells were infected with Ad5 or the ADP mutant pm534 in combination with (A) wild-type AAV or (B) a recombinant AAV vector lacking AAV gene expression. Cell lysis results in lactate dehydrogenase (LDH) release into the culture medium, and LDH catalyzes the conversion of NADH to NAD. Therefore increased NADH oxidation, which was measured by the change in absorbance at 340 nm, was indicative of increased cell lysis. Cells infected with Ad5 alone are shown as white circles, and cells coinfected with Ad5 and AAV are shown as black circles. Black and white triangles represent pm534 infections with and without AAV, respectively. Uninfected cells are shown as white squares, and AAV-infected cells are shown as black squares. LDH assays were conducted in triplicate, and the averages are reported (+/− standard deviation). The data shown are representative of three independent experiments.
Since Rep protein expression has been implicated in the induction of apoptosis, we next determined whether AAV-induced alterations in cell viability require Rep expression. For this purpose, we conducted LDH assays as described above but replaced wild-type AAV with an equal number of recombinant AAV vector (vAVluc) particles (Figure 2B). This replication-defective virus contains the inverted terminal repeat (ITR) elements of the AAV genome within its native capsid, but lacks the Rep and Cap genes. Similar to Figure 2A, extensive cytolysis was observed in Ad5-infected cells, whereas cell lysis of uninfected, vAV-infected, or pm534-infected cells was negligible. Cells infected with both vAV and Ad5 exhibited the same degree of cytolysis as cells infected with Ad5 alone. Thus, addition of the recombinant AAV vector did not alter Ad-induced cytotoxicity. This suggests that the AAV virion is not sufficient to modulate cell viability during coinfection. However in the context of a coinfection with wild type AAV, a role for the capsids or capsid proteins in cytotoxicity cannot yet be formally excluded.
AAV increases Annexin V-PE staining
To test the hypothesis that cell lysis during AAV/pm534 coinfection was the result of apoptosis, we used Annexin V-PE staining followed by flow cytometry. This commonly employed assay takes advantage of the selective binding of Annexin V to phosphatidylserine (PS). In non-apoptotic cells, PS is sequestered in the inner leaflet of the plasma membrane, but when a cell undergoes apoptosis there is a loss of membrane asymmetry that results in the translocation of PS to the cell surface (Hanshaw and Smith, 2005; Lecoeur, de Oliveira-Pinto, and Gougeon, 2002). This exposed PS can be detected with fluorescently labeled Annexin V-PE and flow cytometry. Since membrane permeability is unaffected by PS externalization, vital dyes such as 7-amino-actinomycin D (7-AAD) can be used in conjunction with Annexin V-PE to identify nonviable cells.
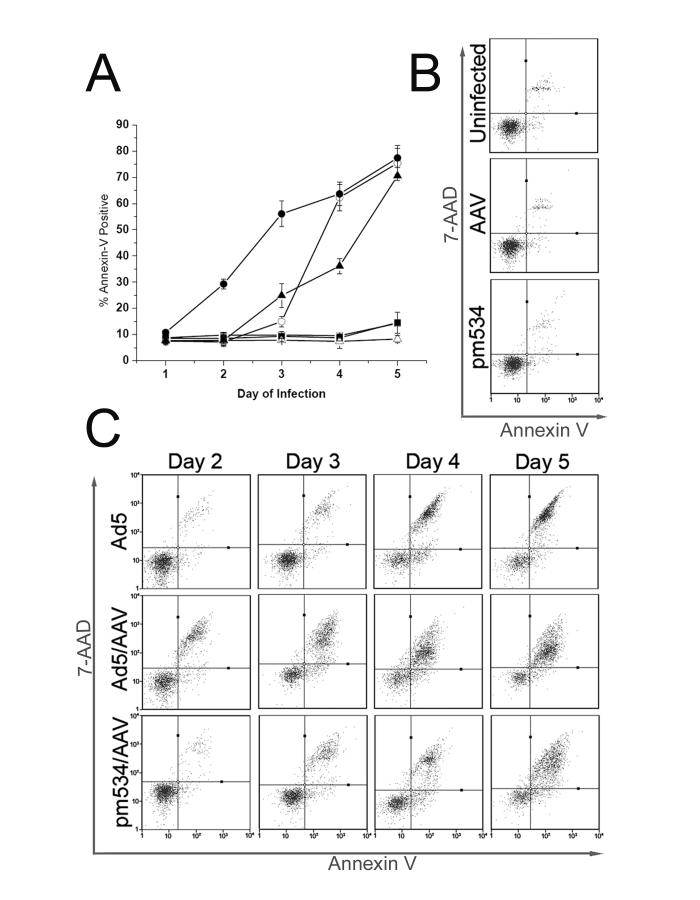
To determine whether AAV induces apoptosis, we infected cells with AAV followed by Ad5 or pm534. Parallel infections were conducted in the absence of AAV, and all infections were performed in triplicate. Twenty-four hours pi, and every 24 hours thereafter for five days, cells were harvested by trypsinization, stained with Annexin V-PE and 7-AAD, and analyzed by flow cytometry. We first examined the percentage of cells that were Annexin V-PE positive, regardless of 7-AAD levels (Figure 3A). This analysis generated a time course study of apoptosis that could be compared to the LDH cell lysis assay (Figure 2). Uninfected cells and cells infected with AAV or pm534 alone did not display appreciable levels of Annexin V-PE staining (Figure 3A). This result is comparable to the LDH cell lysis assays shown above (Figure 2). In samples coinfected with AAV and pm534, Annexin V-PE staining was detected on day 3 (Figure 3A), whereas cell lysis was not identified until day 4 (Figure 2). This shows that AAV/pm534-infected cells were displaying signs of apoptosis prior to cytolysis. In contrast, Annexin V-PE staining of Ad5-infected cells occurred simultaneously with lysis of AAV-coinfected cells (compare Figures 2 and 3A).
Figure 3.
AAV induced apoptosis during coinfection with Ad5 or pm534. Cells were infected, harvested by trypsinization on the indicated day, stained with Annexin V-PE and 7-AAD, and analyzed using the Guava PCA flow cytometer. (A) Time course analysis of Annexin V-PE staining. Flow cytometry results are reported as the percent of Annexin V-PE positive cells (y-axis) on the given day of the infection (x-axis). Ad5-infected cells are depicted as white circles, and Ad5/AAV coinfected cells as black circles. ADP mutant pm534-infected cells are shown as white triangles without AAV and black triangles with AAV. Uninfected cells are depicted by white squares, and AAV-infected cells are represented by black squares. (B) Representative diagrams of Annexin V-PE and 7-AAD staining of uninfected, AAV-infected, and pm534-infected cells. Cells were infected with the virus indicated on the left of each panel and harvested 4 days pi. Fluorescence intensity of Annexin V-PE is represented on the x-axis, and that of 7-AAD is represented on the y-axis. (C) Annexin V-PE and 7-AAD flow cytometry analysis of Ad5-, Ad5/AAV-, and pm534/AAV-infected cells. Cells were harvested between 2-5 days pi, as noted on top of the panels. Fluorescence intensity of Annexin V-PE and 7-AAD are given on the x- and y-axes.
In light of the previous characterizations of Ad-induced cell death as non-apoptotic programmed cell death, we considered the possibility that Annexin V-PE staining of Ad5-infected cells does not reflect PS externalization. For example, loss of membrane integrity could permit Annexin V-PE staining in the absence of apoptosis. There was also considerably less Annexin V-PE staining on Ad5-infected cells than on AAV/Ad5-coinfected cells on days 2 and 3 (Figure 3A). Even though Ad5-induced cell lysis surpassed that of AAV/Ad5 by 4 days pi (Figure 2A); Annexin V-PE staining of Ad5-infected cells never exceeded levels of AAV/Ad5 cell staining (Figure 3A). This concurs with a previous study in which only modest Annexin V-PE staining was observed during Ad infections (Zou et al., 2004). Thus, AAV/pm534 coinfection induced Annexin V-PE staining whereas pm534 alone did not, and AAV/Ad5 induced cell staining sooner than Ad5 infection alone. These results indicate that AAV may induce apoptosis in the presence of a helper virus.
The time course analysis distinguishes necrosis from apoptosis and facilitates a direct comparison between the levels of apoptosis and cell lysis. However, it does not distinguish between early and late apoptosis. We therefore conducted a second data analysis of the flow cytometry assay in which we included the results of both Annexin V-PE and 7-AAD staining. Intact cell membranes of healthy cells exclude 7-AAD, but the dye readily passes through compromised membranes and binds to DNA (Lecoeur, de Oliveira-Pinto, and Gougeon, 2002). Thus, only late apoptotic and necrotic cells are stained with 7-AAD. Based on their resistance to both stains, viable cells are reported in the lower left quadrant of the panels in Figure 3B an 3C. Cells in early apoptosis are in the lower right quadrant, and cells in late apoptosis or early necrosis are in the upper right. In addition to discriminating early and late apoptosis, the inclusion of 7-AAD allowed us to identify distinct populations within the samples. At all assayed time points, there was little or no apoptosis observed in uninfected, AAV-infected, and pm534-infected cells. A representative scatter plot diagram from the fourth day after infection is shown for these samples (Figure 3B). Data from samples that exhibited alterations in cell staining are shown for days 2 through 5 (Figure 3C). Following Ad5 infection alone, there was little cell death or apoptosis on days 1 and 2, but on day 3 there was a sharp increase in late apoptotic/early necrotic cells that resulted in a discrete population of cells in the upper right quadrant. This corresponds with the onset of ADP-induced cell lysis. Cells reported in the upper right quadrant could be undergoing late apoptosis or early necrosis. However, cells in the lower right quadrant are undergoing apoptosis, not necrosis. The presence of early apoptotic cells in Ad5-infected samples was unexpected since wild type Ad-induced cell death has previously been characterized as non-apoptotic programmed cell death (Kanj et al., 2006; Zou et al., 2004). Thus PS externalization, which is commonly used as an indicator of apoptosis, is also present during Ad5-induced cell death.
AAV/pm534-infected cells also began to exhibit Annexin V-PE staining on day 3, but there was a more diffuse pattern of cell staining throughout the assay. Similarly, AAV/Ad5 coinfected cells resulted in a scattered population of stained cells that more closely resembled coinfection with AAV/pm534 than infection with Ad alone. The difference between the cell staining patterns is most apparent on the last assayed time point. On day 5, Annexin V-PE staining alone indicated little difference in the percentage of apoptotic cells among Ad5, AAV/Ad5 and AAV/pm534 infections. However, the addition of 7-AAD revealed an interesting pattern of cell staining. Ad5 infection produced a discrete population of late apoptotic cells, but AAV coinfection with either Ad5 or pm534 resulted in a more diffuse pattern of cell staining. This likely reflects ADP-induced apoptosis observed in Ad5-infected cells. The significant reduction in ADP expression in an AAV/Ad5 infection (Figure 1) and the lack of ADP in pm534-infected cells suggests that the cell staining pattern observed in the AAV-infected cells in Figure 3C is due to AAV-mediated cellular changes. These results suggest that AAV induces cell death and membrane asymmetry during coinfection with a helper virus.
AAV-induced Annexin V-PE staining is reduced by a pan-caspase inhibitor
While Annexin V-PE staining is a valuable tool for characterizing cell death, additional assays are required to differentiate between apoptosis and non-apoptotic programmed cell death. Caspase activation and changes in cell morphology are hallmarks of apoptosis. Based on these characteristics, adenovirus-induced cell death is classified as non-apoptotic programmed cell death even though Annexin V-PE staining was observed. Therefore, to determine whether AAV induced apoptosis, we next assayed infected cells for caspase activation using two methods: a caspase inhibitor (ZVAD) and a caspase substrate (Ac-DEVD-AMC). First, infected cells were treated with the general caspase inhibitor ZVAD. We then assayed them for Annexin V-PE-staining using the flow cytometry-based method described above. If viral infection induced caspase activation, there would be a decrease in the percentage of Annexin V-PE stained cells following ZVAD treatment relative to untreated cells. The tetrapeptide was solubilized in DMSO, which can induce apoptosis, so cells were either treated with ZVAD or an equivalent amount of DMSO. Cells were infected with AAV and/or either strain of adenovirus and treated with ZVAD or DMSO shortly thereafter. Additional caspase inhibitor and DMSO were added at 48 hours pi to control for potential protease digestion. Seventy-two hours pi, the cells were harvested, stained, and analyzed by flow cytometry. The average percentage of Annexin V-PE positive cells is recorded in Table 1 along with the statistical significance of the difference between ZVAD and DMSO treatments. AAV or pm534 infection in the presence of either ZVAD or DMSO induced levels of Annexin V-PE staining that were indistinguishable from uninfected cells. In Ad5-infected cells, the addition of the caspase inhibitor did not alter the percentage of Annexin V-PE positive cells. This indicates that the Annexin staining associated with Ad5 infection was not dependent on caspase activation thus confirming the results of previous studies (Kanj et al., 2006; Zou et al., 2004). In contrast, ZVAD treatment decreased the level of Annexin V-PE staining that was observed during a productive AAV infection, regardless of whether the helper virus was Ad5 or pm534. While this reduction is modest, it represents a significant decrease in cell killing as a result of caspase inhibition. This suggests that AAV-induced cell death is, to some degree, mediated through caspase activation.
Table 1.
The caspase inhibitor ZVAD reduced Annexin V-PE staining of AAV/Ad5 or AAV/pm534 coinfected cells. Cultured cells were infected with Ad5 (moi 10), pm534 (moi 10) and/or AAV (IU 100). Two hours pi, cells were treated with DMSO or 8 100μM ZVAD dissolved in DMSO. Fresh ZVAD was added to the media 48h pi. Cells were harvested 72 to 80 h pi, stained with Annexin V-PE and 7-AAD, and assayed by flow cytometry. Infections were conducted in triplicate on two separate occasions. Values are reported as the average number of Annexin V-PE positive cells (+/− standard deviation) from both assays. The p values indicate the statistical significance of differences between ZVAD treatment and DMSO treatment for each virus or combination of viruses based on independent t-test analysis.
| DMSO | ZVAD | p-value | |
|---|---|---|---|
| Uninfected | 11.8± 2.7 | 11.4 ± 1.9 | 0.769 |
| AAV | 9.7 ± 3.1 | 12.3 ± 5.9 | 0.358 |
| Ad5 | 41.5 ± 9.0 | 43.2 ± 7.9 | 0.734 |
| Ad5/AAV | 70.2 ± 9.1 | 56.6 ± 6.3 | 0.014 |
| pm534 | 13.5 ± 1.6 | 14.7 ± 4.6 | 0.572 |
| pm534/AAV | 39.2 ± 4.1 | 30.6 ± 5.6 | 0.012 |
AAV coinfection mediates caspase activation
To confirm these results, we tested cell lysates for functional caspase activity using the caspase 3- and 7-specific substrate Ac-DEVD-AMC. This peptide-coumarin conjugate is non-fluorescent until the coumarin is cleaved from the peptide by active caspases, so increased fluorescence is indicative of caspase activity. To determine whether AAV confection with a helper virus resulted in caspase activation, cells were infected as described above and harvested 4 days pi. Cell lysates were incubated with Ac-DEVD-AMC at 37°C, and the fluorescence was measured 1 h pi. As a positive control for apoptosis, cells were treated with the pro-apoptotic agent doxorubicin for 48h. As shown in Figure 5, cells infected with AAV, Ad5, or pm534 alone did not exhibit discernible caspase activity. In the presence of either Ad5 or pm534, AAV induced significant caspase activation, which suggests that AAV mediates caspase-dependent apoptosis. However, AAV-infected cells demonstrated substantially less caspase activity than cells treated with doxorubicin. Together with the incomplete inhibition of AAV-mediated apoptosis by ZVAD (Table 1), this suggests that AAV induces both caspase –dependent and caspase-independent apoptosis.
Figure 5.
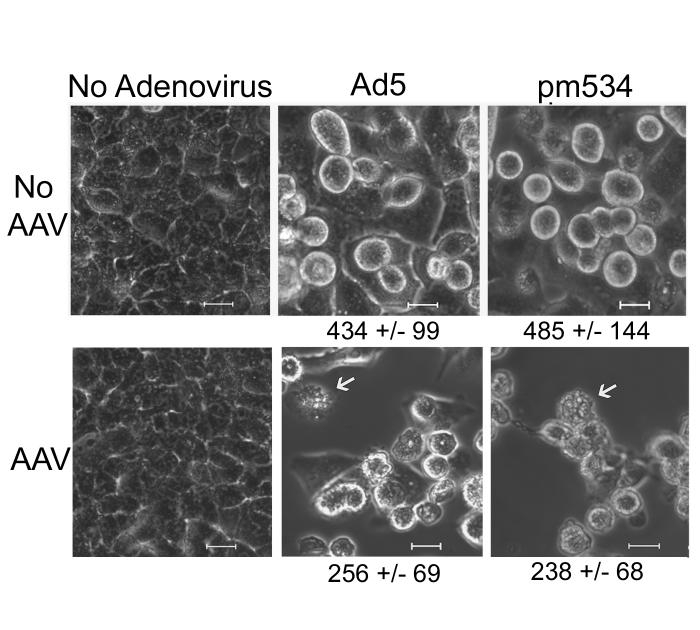
Helper virus-supported AAV infections alter cell morphology. A549 cells were infected with AAV (100 IU) and/or Ad5 (5 moi) or pm534 (5 moi). Unfixed cells were microscopically examined 3 days pi. Uninfected cells and cells infected with Ad alone are on the top panels, and the corresponding infections with the addition of AAV are below. Cells infected with either Ad5 or pm534 alone were rounded up and enlarged, but cells infected with a helper virus and AAV exhibited cell shrinkage and membrane blebbing. The arrows indicate cells with extensive membrane blebbing. The average area of cells exhibiting cytopathic effect is shown in nm2.
AAV coinfection induces apoptotic cell morphology
The assays above distinguish apoptosis based on biochemical alterations that are central to the classification of cell killing as apoptosis or non-apoptotic programmed cell death. Another hallmark of apoptosis is the changes in cell morphology that accompany the biochemical phenomena. Apoptotic cells become smaller and their membranes “bleb,” whereas necrotic cells swell and burst. Therefore, to further test the hypothesis that a productive AAV infection induces apoptosis, we examined the morphology of infected cells. Cell monolayers were infected with Ad5 or pm534 in the presence or absence of AAV. Cell morphology of unfixed cells was microscopically examined 3 days pi. As shown in Figure 5, uninfected and AAV-infected cells were confluent but displayed little CPE. Previous studies report that cells infected with ADP mutants exhibit CPE comparable to wild-type Ad even though they do not lyse (Tollefson et al., 1996a; Zou et al., 2004). Our assays confirm these reports since cells infected with pm534 were swollen, rounded-up and the plasma membranes had a smooth appearance without signs of blebbing. Ad5-infected cells exhibited similar morphology as pm534-infected until nearly 4 days pi. At that time, the Ad5-infected cells began to lyse, while the pm534 remained intact (data not shown). Coinfection with AAV and Ad5 or pm534 induced significant alterations in cell morphology. As opposed to the smooth appearance of Ad-infected cells, cells infected with AAV and either helper virus displayed extensive membrane blebbing. This supports the hypothesis that AAV induces apoptosis during coinfection with Ad.
During our examination of cell morphology, it appeared that cells coinfected with AAV were smaller than cells infected with Ad alone, and cell shrinkage is a characteristic of apoptosis. Therefore, to quantify cell size and determine whether AAV induced significant cell shrinkage, we measured the two-dimensional area of cells exhibiting CPE. In brief, we captured multiple microscopic images of infected cells, including those shown in Figure 5. We then traced the circumference of up to 100 cells from each population using the add measurement/region area setting on the Spot Advance microanalysis software. Only cells exhibiting CPE were included. The area of each outlined cell was calculated, and the resulting data were averaged and analyzed by independent t-test. The average cell sizes are noted under the corresponding images in Figure 5. There was a statistically significant decrease in cell size during pm534/AAV coinfections compared to pm534 alone. Similar results were observed Ad5/AAV-infected cells versus Ad5 alone. In both analyses, the p values approached zero. This indicates that productive AAV infection in the presence of a helper virus induces cell shrinkage, which is a hallmark of apoptosis. Taken together, the data presented in this report suggest that AAV mediates apoptosis based on both biochemical and morphological alterations in the cell.
Viral infections alter the pH of cell culture media
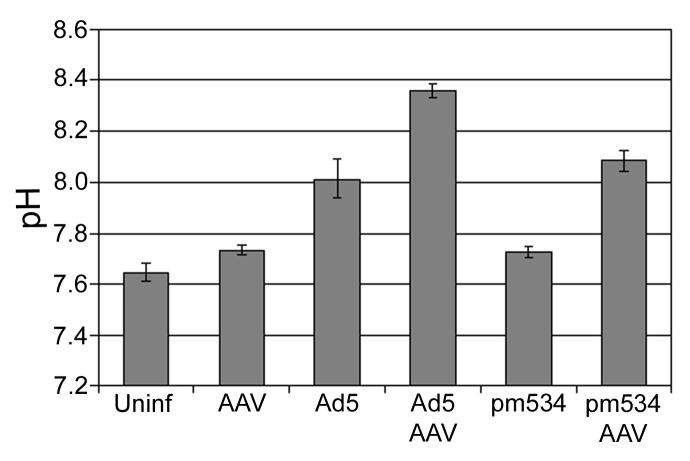
During the course of our studies, we noticed that productive AAV infections altered the pH of the media to such a degree that it was visible during a cursory examination of the cell cultures. This effect was apparent by day 3 and became more prominent by day 6 of the infections. Changes in extracellular pH are generally not obvious during standard Ad preparations and assays since investigators typically harvest cells at 2 to 3 days pi. To quantify AAV-mediated alterations in pH, we infected cells with either strain of Ad and/or AAV and measured the pH of the media after incubating the cells 6 days at 37°C. Infections were conducted in triplicate, and the averages are depicted in Figure 6. The statistical significance of the difference in pH between cells infected with adenovirus and AAV versus cells infected with adenovirus alone is also noted above the appropriate bar. The media pH from uninfected, AAV-infected, and pm534-infected cells ranged from 7.6-7.7 by 6 days pi. Ad5 alone prevented the acidification of the medium, resulting in a pH of 8.01 on day 6, and the addition of AAV with Ad5 infection further increased pH to 8.36. The pH during pm534 infections was also buffered by coinfection with AAV. As a result, the media has a pH of 8.08 on day six of pm534/AAV coinfections. These data suggest that productive AAV infection increases the pH, thereby preventing the acidification of media that usually occurs during a lengthy incubation. Although the role of extracellular pH in AAV-induced apoptosis is unclear, previous reports indicate that changes in pH contribute to the induction of apoptosis by various agents. For example, photodynamic treatment preferentially induced apoptosis in colon adenocarcinoma cells when the media was maintained at pH 7.4, but necrotic cell death was more common with a pH of 6.5 (Sharma et al., 2005). Another report indicates that low pH promotes TRAIL-induced apoptosis in human prostate and colorectal carcinoma cells (Lee et al., 2004). Intracellular acidification has also been associated with apoptosis (Goossens et al., 2000; Gottlieb et al., 1996). Identifying the role of pH in AAV-induced apoptosis is outside the scope of this report. Nonetheless, these studies provide the first report of AAV-induced caspase-dependent and caspase–independent apoptosis during coinfection with Ad.
Figure 6.
Viral infections alter extracellular pH. Approximately 4.5 × 105 cells were infected with 100 IU of AAV and/or 10 moi of Ad5 or pm534. Six days pi, the media was removed from each well and measured using a standard pH meter. The assay was conducted in triplicate, and the average pH values are reported (+/− standard deviation).
DISCUSSION
Previous studies suggest that Rep expression is sufficient to induce caspase-dependent apoptosis in cultured cells. In this report, we examined AAV-mediated alterations in cell viability in the context of a fully permissive infection. AAV increased cell lysis during the first three days of coinfection with Ad5 but reduced it thereafter. A parallel effect on Annexin V-PE-staining was observed. This contrast between increased cell killing during the early stages of Ad5 coinfection and decreased cell death late in the infection may reflect the respective effects of AAV's induction of apoptosis and its reduction of ADP expression. However, dissecting the effects of decreased ADP expression versus AAV-mediated cell killing during Ad5 coinfection is hampered by the current lack of knowledge of the mechanisms of ADP-induced cell death. By utilizing an ADP mutant, we were able to eliminate ADP-related alterations in cell viability while retaining the necessary helper functions to support a productive AAV infection. Using this system, we examined the role of AAV-mediated cell killing during coinfection. Our results indicate that AAV induced cell killing, loss of membrane symmetry, caspase activation, changes in cell morphology, and alterations in media pH. During coinfection with wild-type Ad5, the effects of AAV on cell viability were more complex since both viruses mediate cell killing. Nonetheless, we did not detect caspase activation in Ad5-induced cell death, so caspase activation during Ad5/AAV coinfection is likely mediated by AAV.
In a previous study, expression of AAV Rep proteins following plasmid transfection induces p53-independent apoptosis that could be eliminated by the pan-caspase inhibitor ZVAD (Schmidt, Afione, and Kotin, 2000). We observed similar alterations in the cell during our study of AAV coinfections. This suggests that the apoptosis observed during AAV coinfections may be induced by Rep proteins. The role of Rep proteins in AAV-induced cell death is also supported by the lack of cell lysis induction a recombinant AAV vector that lacks Rep expression (Figure 2B).
While caspase activation and ZVAD inhibition indicate that AAV modulates caspase-dependent cell signaling pathways, the modest degree of in ZVAD-mediated inhibition suggests that AAV also utilizes caspase-independent mechanisms to induce cell death. The details of this mechanism remain unclear. It is possible that AAV and its Rep proteins induce apoptosis through direct effects on cellular gene expression and signaling. Alternatively, modulation of Ad gene expression by AAV could be responsible. There is evidence supporting both mechanisms. AAV infection in the absence of Ad can cause significant changes in the host cell including decreased cellular proliferation (Bantel-Schaal, 1990; Hermanns et al., 1997) and induction of a late S and/or G2 phase cell block by the inhibition of retinoblastoma (Rb) phosphorylation (Hermanns et al., 1997). AAV also alters the expression of proteins involved in cell differentiation (Klein-Bauernschmitt, zur Hausen, and Schlehofer, 1992), DNA repair (Winocour et al., 1992) and cell cycle regulation (Hermanns et al., 1997). AAV infection enhances cisplatin-induced apoptosis (Duverger et al., 2002). Ectopic expression of the Rep proteins inhibits cell proliferation and gene expression from a variety of transcription promoters (Muzyczka and Berns, 2001). When a Rep-expressing cell line was stressed by DNA damage, p53-independent apoptosis resulted (Zhou and Trempe, 1999; Zhou, Yang, and Trempe, 1999). Rep78 induced apoptosis in wild type p53-containing human embryonal carcinoma NT-2 cells and in p53-null promyelocytic HL-60 cells (Schmidt, Afione, and Kotin, 2000). Rep78 and Rep68 expression have also been reported to arrest cells by associating with, and inhibiting expression of, Cdc25A (Berthet et al., 2005). Rep78 and Rep68 were also reported to induce a DNA damage response via nicking of chromosomal DNA (Berthet et al., 2005). Given the extent of AAV and Rep effects on the cell, apoptosis may be a common result in AAV infections.
AAV could also initiate apoptosis and alter cell viability through its effects on Ad gene expression. There are several Ad proteins that are expressed during the early phase of viral infections that can modulate apoptotic pathways (reviewed in (Gallimore and Turnell, 2001; Shenk, 2001)). For example, in the process of activating gene transcription and altering the cellular environment to support viral replication, E1A proteins induce p53-dependent and p53–independent apoptosis. This is mitigated by the actions of the E1B-19K, E1B-55K, and E4orf6 proteins. E1B-55K binds to p53 and restricts its function, while the E1B-55K/E4orf6 complex targets p53 to the proteosomes for degradation. E1B-19K functions as a BCL-2 homolog and blocks both p53-dependent and p53-independent apoptosis. Exogenous expression of E4orf4 is capable of inducing p53-independent apoptosis in cultured cancer cells, and ADP mediates cytolysis through an undetermined mechanism. To prevent cell death during the initial stages of infection yet induce cytolysis at the end of the replicative cycle, it is imperative that a balance is maintained between the proteins that induce apoptosis and those that suppress it. Since AAV exerts different degrees of inhibition on individual transcription units (Timpe, Verrill, and Trempe, 2006), it stands to reason that this may introduce an imbalance in apoptosis regulation. Thus, AAV could alter cell viability and apoptosis via modulation of Ad gene expression.
The role of extracellular pH modulation during AAV coinfection is undetermined yet potentially significant. Numerous studies correlate cell death with alterations in extracellular and intracellular pH. For example, increased extracellular pH in the absence of any other cytotoxic agents was sufficient to induce caspase-dependent apoptosis in cultured endothelial cells, and this increase in pH was accompanied by a corresponding increase in intracellular pH (Cutaia et al., 2005). In another report, elevated intracellular pH was associated with increased mitochondrial membrane potential, reactive oxygen species, DNA fragmentation, and cell death (Majima et al., 1998). Even minor changes in pH correlate with alterations in cell proliferation, Ca2+ signaling, and apoptosis (reviewed in (Schreiber, 2005)) Therefore, changes in pH during AAV coinfection may play a significant role in viral-induced apoptosis. Future studies comparing intracellular and extracellular pH during productive AAV infections in conjunction with an examination of Ca2+-signaling pathways and mitochondrial membrane potential will likely provide insight into the effects of AAV on apoptosis. Clearly, defining the mechanisms of AAV-induced cell death will be challenging, and it is complicated by AAV's dependence on a helper virus for a productive infection. To minimize the effects of Ad on cell viability, we utilized the ADP mutant pm534 to provide the necessary helper functions for AAV. Our data indicate that productive AAV infection resulted in cell death, loss of membrane asymmetry, caspase activation, and alterations in cell morphology. These studies demonstrate for the first time that AAV infection induces caspase-dependent and caspase-independent apoptosis.
MATERIALS AND METHODS
Cells and Viruses
A549 cells (human lung carcinoma; ATCC CCL-185) were grown as monolayers in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 6 to 10% fetal bovine serum, penicillin (50 mg/ml), streptomycin (50 mg/ml), gentamicin (100 μg/ml), and amphotericin B (2.5 μg/ml). Adeno-associated virus serotype 2 (AAV) was generated by pNTC244 transfection of adenovirus-infected HeLa monolayers as previously reported (Casper et al., 2005). AAV was purified over CsCl or by heparin-agarose chromatography and titered by indirect immunofluorescence (Laughlin et al., 1979) or by a limiting dilution assay (King et al., 2001). The AAV2-based recombinant vector vAVluc, which contains a luciferase gene in place of the Rep genes, was generated as previously described (Smith, Collaco, and Trempe, 2003). Adenovirus type 5 (Ad5), which was originally obtained from ATCC, was propagated, purified, and titered as previously reported (Winters and Russell, 1971). Drs. William Wold and Ann Tollefson graciously provided the Ad5-derived ADP mutant pm534.
Immunoblots
Rabbit antiserum against E3 ADP protein was generously provided by Dr. William Wold. Affinity-purified, polyclonal AAV Rep-specific antibody was obtained from rabbits immunized with E. coli-expressed recombinant Rep and Cap proteins and probed with anti-rabbit (Pierce, 31340) alkaline phosphatase-conjugated secondary antibody.
Lactate Dehydrogenase (LDH) Assay
Approximately 1.5 × 105 A549 cells were seeded into each well of 12-well tissue culture plates and incubated overnight at 37°C. The next day, the cells were infected in serum-free media with 100 infectious units (IU) of AAV or an equal number of vAVluc particles. After incubating one hour at 37°C, 5 multiplicities of infection (moi) of Ad5 or pm534 were added to the wells. One hour post-Ad infection (pi), the infectious media was replaced with 1 ml DMEM containing 7% FBS per well. The cells were incubated 24h, tested for LDH activity, and reported as Day One. The assay was repeated every 24h for 6 days. To measure LDH activity, 20μl of media was removed from each well and combined with 480μl of LDH reaction mixture (50 mM TrisHCl pH 8, 0.2 mM NADH, and 0.6 mM pyruvate). The absorbance at 340nm was determined every 12 seconds for 10 min on a Beckman Coulter DU640 spectrophotometer. The oxidation of NADH by LDH was calculated based on the rate of change in absorbance and the NADH extinction coefficient of 6.22. The measurements are reported as μmole NADH oxidized per minute per well. Three independent assays were conducted in triplicate, and the average values of a representative experiment are reported with standard deviations.
Annexin V-PE Staining
A549 cells at approximately 70% confluency were infected with 100 IU AAV followed by 10 moi of Ad5 or pm534 as described above. The cells were harvested by trypsinization and assayed at the given times. Annexin V-PE and 7-AAD staining was conducted using the GUAVA Nexin Kit (catalog # 4500-0010) by Guava Technologies following the manufacturer's protocol with modification. Each sample of 1 × 105 cells was incubated with only 1μl Annexin V-PE, 1μl Nexin 7-AAD and 10μl 1X Nexin buffer. Cell staining was measured using a GUAVA PCA personal flow cytometer and analyzed using Cytosoft version 2.1.4 software. The samples were assayed in triplicate on three separate occasions. In figure 3A, values are reported as the average number of Annexin V-PE positive cells (+/− standard deviation) from one assay. In Figure 3B and 3C, representative diagrams are shown for one of three independent experiments.
Annexin V-PE Staining with Caspase Inhibitor
Infections were conducted in 48-well plates as described above with the addition of benzyloxycarbonyl-Val-Ala-Aso (OMe)fluoromethyl ketone (ZVAD; 100 μfinal concentration) or an equivalent amount of DMSO at 2 h pi. Additional ZVAD or DMSO was added 48 h pi. Cells were harvested 72 to 80 h pi, stained, and assayed by flow cytometry. Infections were conducted in triplicate on two separate occasions. In Table 1, values are reported as the average number of Annexin V-PE positive cells (+/− standard deviation) from both assays. The given p values were determined using independent t-test analysis and SPSS 13.0 software.
Fluorogenic Caspase Substrate
Caspase activity was measured using the fluorogenic substrate Ac-DEVD-AMC (7-acetyl-Asp-Glu-Val-Asp-amino-4-methylcoumarin; Calbiochem catalog # 235425). Nearly confluent A549 cells in 6 well tissue culture plates were infected with AAV (100 IU) and Ad5 or pm534 as described above. As a positive control for apoptosis, uninfected cells were treated with 20 μg/ml doxorubicin. Four days pi, the cells were harvested by trypsinization, washed with cold PBS, and resuspended in 50μl cell lysis buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 1mM DTT, 100 μM EDTA). After incubating 20 min on ice, the cell lysates were centrifuged at 10,000 × g, 10 min at 4°C. Protein concentrations of the cell lysate supernatants were measured spectrophotometrically using a detergent-competent system from BioRad (500-0113). Equal amounts of protein in 50 μl of assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 100μM EDTA, 10% glycerol) per well was added to a black 96-well plate. Cell lysate supernatants containing equal amounts of protein in a final volume of 50 μl were added next, followed by 5 μl of 1 mM Ac-DEVD-AMC. The reaction mixture was incubated for 1 h at 37°C, and fluorescence was measured with a Packard Bioscience Fusion α (Perkins Elmer) using excitation 365 nm and emission 460 nm.
Cell Morphology
A549 cells were infected in 48-well plates with AAV (100 IU) and Ad5 (5 moi) or pm534 (5 moi). Unfixed cells were microscopically examined 3 days pi using an Olympus IX70 microscope and a 30X phase contrast objective. The average area of cells exhibiting cytopathic effect was measured using the region area measurement feature of the Spot Advanced MacOS version 4.0.9 software. The two-dimensional area of 50 to 100 cells from each infection were averaged and analyzed by independent t-test using SPSS version 13.0 software
Extracellular pH
A549 cells were infected in 6 well plates with AAV (100 IU) and/or 10 moi Ad5 or pm534 as described above and incubated in 1 ml DMEM for 6 days. The media was removed from the wells and measured using a ThermoOrion model 420 pH meter. Three independent experiments were conducted. The reported values indicate the average pH for triplicate samples +/− standard deviation from one experiment. The p-values were calculated using the independent t-test and SPSS 13.0 software.
Figure 4.
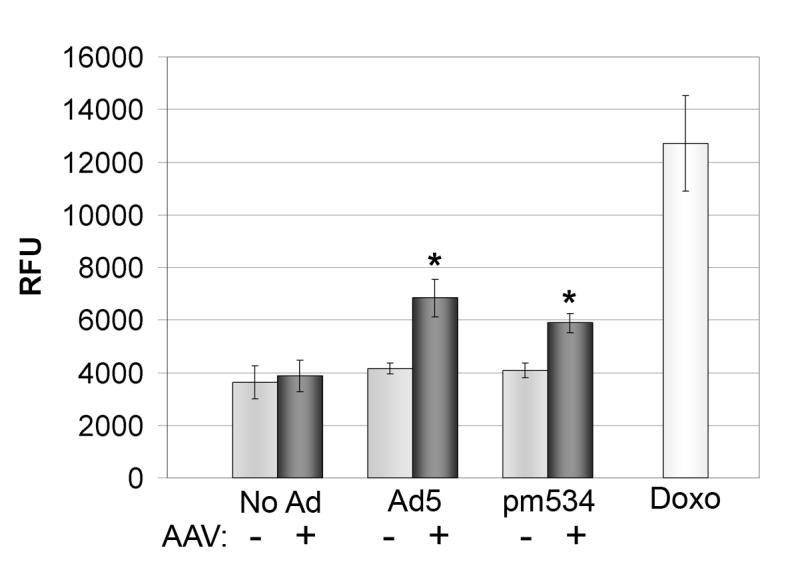
Coinfection with AAV and Ad induces caspase activation. Approximately 1 × 106 cells were infected with AAV and/or pm534 or Ad5. Cells were harvested 4 days pi, and equal amounts of cell lysates were incubated 1 hour with the fluorogenic caspase substrate Ac-DEVD-AMC. Fluorescence was reported in arbitrary relative fluorescence units (RFU) as an indicator of caspase activation. Uninfected and doxorubicin-treated cells were included as negative and positive controls. Each bar indicates the average of 6 samples with the indicated standard deviation. The data shown are representative of three independent experiments. There was no statistical difference in average caspase activity of uninfected cells compared with AAV-infected cells (p = 0.437). AAV in the presence of either Ad5 or pm534 significantly increased caspase activation relative to that of the corresponding adenovirus alone, (p ≤ 0.001).
ACKNOWLEDGEMENTS
We would like to thank Drs. Anne Tollefson and William Wold for providing us the pm534 mutant, ADP antisera and numerous helpful suggestions. This work was supported in part by NIH GM64765 (JPT), AI51471(JPT) and AI64129 (JMT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bantel-Schaal U. Adeno-associated parvoviruses inhibit growth of cells derived from malignant human tumors. Int J Cancer. 1990;45(1):190–4. doi: 10.1002/ijc.2910450134. [DOI] [PubMed] [Google Scholar]
- Berthet C, Raj K, Saudan P, Beard P. How adeno-associated virus Rep78 protein arrests cells completely in S phase. Proc. Natl. Acad. Sci. USA. 2005;102:13634–13639. doi: 10.1073/pnas.0504583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper JM, Timpe JM, Dignam JD, Trempe JP. Identification of an adeno-associated virus Rep protein binding site in the adenovirus E2a promoter. J Virol. 2005;79(1):28–38. doi: 10.1128/JVI.79.1.28-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto BC, Armstrong JA, Atchison RW, Hammon WM. Studies on the relationship between adeno-associated virus type 1 (AAV-1) and adenoviruses. II. Inhibition of adenovirus plaques by AAV; its nature and specificity. Virology. 1967;33(3):452–8. doi: 10.1016/0042-6822(67)90120-1. [DOI] [PubMed] [Google Scholar]
- Casto BC, Atchison RW, Hammon WM. Studies on the relationship between adeno-associated virus type I (AAV-1) and adenoviruses. I. Replication of AAV-1 in certain cell cultures and its effect on helper adenovirus. Virology. 1967;32(1):52–9. doi: 10.1016/0042-6822(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Cutaia M, Black AD, Cohen I, Cassai ND, Sidhu GS. Alkaline stress-induced apoptosis in human pulmonary artery endothelial cells. Apoptosis. 2005;10(6):1457–67. doi: 10.1007/s10495-005-1402-5. [DOI] [PubMed] [Google Scholar]
- Duverger V, Sartorius U, Klein-Bauernschmitt P, Krammer PH, Schlehofer JR. Enhancement of cisplatin-induced apoptosis by infection with adeno-associated virus type 2. Int J Cancer. 2002;97(5):706–12. doi: 10.1002/ijc.10077. [DOI] [PubMed] [Google Scholar]
- Gallimore PH, Turnell AS. Adenovirus E1A: remodelling the host cell, a life or death experience. Oncogene. 2001;20(54):7824–35. doi: 10.1038/sj.onc.1204913. [DOI] [PubMed] [Google Scholar]
- Goossens JF, Henichart JP, Dassonneville L, Facompre M, Bailly C. Relation between intracellular acidification and camptothecin-induced apoptosis in leukemia cells. Eur J Pharm Sci. 2000;10(2):125–31. doi: 10.1016/s0928-0987(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Nordberg J, Skowronski E, Babior BM. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc Natl Acad Sci U S A. 1996;93(2):654–8. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanshaw RG, Smith BD. New reagents for phosphatidylserine recognition and detection of apoptosis. Bioorg Med Chem. 2005;13(17):5035–42. doi: 10.1016/j.bmc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Hermanns J, Schulze A, Jansen-Db1urr P, Kleinschmidt JA, Schmidt R, zur Hausen H. Infection of primary cells by adeno-associated virus type 2 results in a modulation of cell cycle-regulating proteins. J Virol. 1997;71(8):6020–7. doi: 10.1128/jvi.71.8.6020-6027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz CA, Torres V, Quest AF. Beyond apoptosis: nonapoptotic cell death in physiology and disease. Biochem Cell Biol. 2005;83(5):579–88. doi: 10.1139/o05-065. [DOI] [PubMed] [Google Scholar]
- Jing XJ, Kalman-Maltese V, Cao X, Yang Q, Trempe JP. Inhibition of adenovirus cytotoxicity, replication, and E2a gene expression by adeno-associated virus. Virology. 2001;291(1):140–51. doi: 10.1006/viro.2001.1192. [DOI] [PubMed] [Google Scholar]
- Kanj SS, Dandashi N, El-Hed A, Harik H, Maalouf M, Kozhaya L, Mousallem T, Tollefson AE, Wold WS, Chalfant CE, Dbaibo GS. Ceramide regulates SR protein phosphorylation during adenoviral infection. Virology. 2006;345(1):280–9. doi: 10.1016/j.virol.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Khleif SN, Myers T, Carter BJ, Trempe JP. Inhibition of cellular transformation by the adeno-associated virus rep gene. Virology. 1991;181:738–741. doi: 10.1016/0042-6822(91)90909-u. [DOI] [PubMed] [Google Scholar]
- King JA, Dubielzig R, Grimm D, Kleinschmidt JA. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. Embo J. 2001;20(12):3282–91. doi: 10.1093/emboj/20.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Bauernschmitt P, zur Hausen H, Schlehofer JR. Induction of differentiation-associated changes in established human cells by infection with adeno-associated virus type 2. J Virol. 1992;66(7):4191–200. doi: 10.1128/jvi.66.7.4191-4200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin CA, Myers M, Risin DL, Carter BJ. Defective-interfering particles of the human parvovirus adeno-associated virus. Virology. 1979;94:162–174. doi: 10.1016/0042-6822(79)90446-x. [DOI] [PubMed] [Google Scholar]
- Lecoeur H, de Oliveira-Pinto LM, Gougeon ML. Multiparametric flow cytometric analysis of biochemical and functional events associated with apoptosis and oncosis using the 7-aminoactinomycin D assay. J Immunol Methods. 2002;265(12):81–96. doi: 10.1016/s0022-1759(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Song JJ, Kim JH, Kim HR, Song YK. Low extracellular pH augments TRAIL-induced apoptotic death through the mitochondria-mediated caspase signal transduction pathway. Exp Cell Res. 2004;293(1):129–43. doi: 10.1016/j.yexcr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Majima HJ, Oberley TD, Furukawa K, Mattson MP, Yen HC, Szweda LI, Clair DK. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem. 1998;273(14):8217–24. doi: 10.1074/jbc.273.14.8217. [DOI] [PubMed] [Google Scholar]
- Muzyczka N, Berns KI. Parvoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed. Vol. 2. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2327–2359. 2 vols. [Google Scholar]
- Nada S, Trempe JP. Characterization of adeno-associated virus rep protein inhibition of adenovirus E2a gene expression. Virology. 2002;293(2):345–55. doi: 10.1006/viro.2001.1286. [DOI] [PubMed] [Google Scholar]
- Ostrove JM, Duckworth DH, Berns KI. Inhibition of adenovirus-transformed cell oncogenicity by adeno-associated virus. Virology. 1981;113:521–533. doi: 10.1016/0042-6822(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Schlehofer JR. The tumor suppressive properties of adeno-associated viruses. Mutat Res. 1994;305(2):303–13. doi: 10.1016/0027-5107(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Afione S, Kotin RM. Adeno-associated virus type 2 Rep78 induces apoptosis through caspase activation independently of p53. J. Virol. 2000;74:9441–9450. doi: 10.1128/jvi.74.20.9441-9450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol. 2005;205(3):129–37. doi: 10.1007/s00232-005-0778-z. [DOI] [PubMed] [Google Scholar]
- Sharma M, Sahu K, Dube A, Gupta PK. Extracellular pH influences the mode of cell death in human colon adenocarcinoma cells subjected to photodynamic treatment with chlorin p6. J Photochem Photobiol B. 2005;81(2):107–13. doi: 10.1016/j.jphotobiol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Shenk T. Adenoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed. Vol. 2. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2265–2300. 2 vols. [Google Scholar]
- Smith AD, Collaco RF, Trempe JP. Enhancement of recombinant adeno-associated virus type 2-mediated transgene expression in a lung epithelial cell line by inhibition of the epidermal growth factor receptor. J Virol. 2003;77(11):6394–404. doi: 10.1128/JVI.77.11.6394-6404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpe JM, Verrill KC, Trempe JP. Effects of Adeno-Associated Virus on Adenovirus Replication and Gene Expression during Coinfection. Journal of Virology. 2006;80(16) doi: 10.1128/JVI.00198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Ryerse JS, Scaria S, Hermiston TW, Wold WSM. The E3-11.6kDa adenovirus death protein (ADP) is required for efficient cell death; Characterization of cells infected with adp mutants. Virology. 1996a;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WSM. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virology. 1996b;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Saha SK, Wold WS. The 11,600-MW protein encoded by region E3 of adenovirus is expressed early but is greatly amplified at late stages of infection. J Virol. 1992;66(6):3633–42. doi: 10.1128/jvi.66.6.3633-3642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E, Puzis L, Etkin S, Koch T, Danovitch B, Mendelson E, Shaulian E, Karby S, Lavi S. Modulation of the cellular phenotype by integrated adeno-associated virus. Virology. 1992;190:316–329. doi: 10.1016/0042-6822(92)91218-j. [DOI] [PubMed] [Google Scholar]
- Winters WD, Russell WC. Studies on the assembly of adenovirus in vitro. J. Gen. Virol. 1971;10:181–194. doi: 10.1099/0022-1317-10-2-181. [DOI] [PubMed] [Google Scholar]
- Zhou C, Trempe JP. Induction of apoptosis by cadmium and the adeno-associated virus Rep proteins. Virology. 1999;261:280–287. doi: 10.1006/viro.1999.9882. [DOI] [PubMed] [Google Scholar]
- Zhou C, Yang Q, Trempe JP. Enhancement of UV-induced cytotoxicity by the adeno-associated virus replication proteins. Biochem. Biophys. ACTA. 1999;1444:371–383. doi: 10.1016/s0167-4781(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Zou A, Atencio I, Huang WM, Horn M, Ramachandra M. Overexpression of adenovirus E3-11.6K protein induces cell killing by both caspase-dependent and caspase-independent mechanisms. Virology. 2004;326(2):240–9. doi: 10.1016/j.virol.2004.06.007. [DOI] [PubMed] [Google Scholar]