Abstract
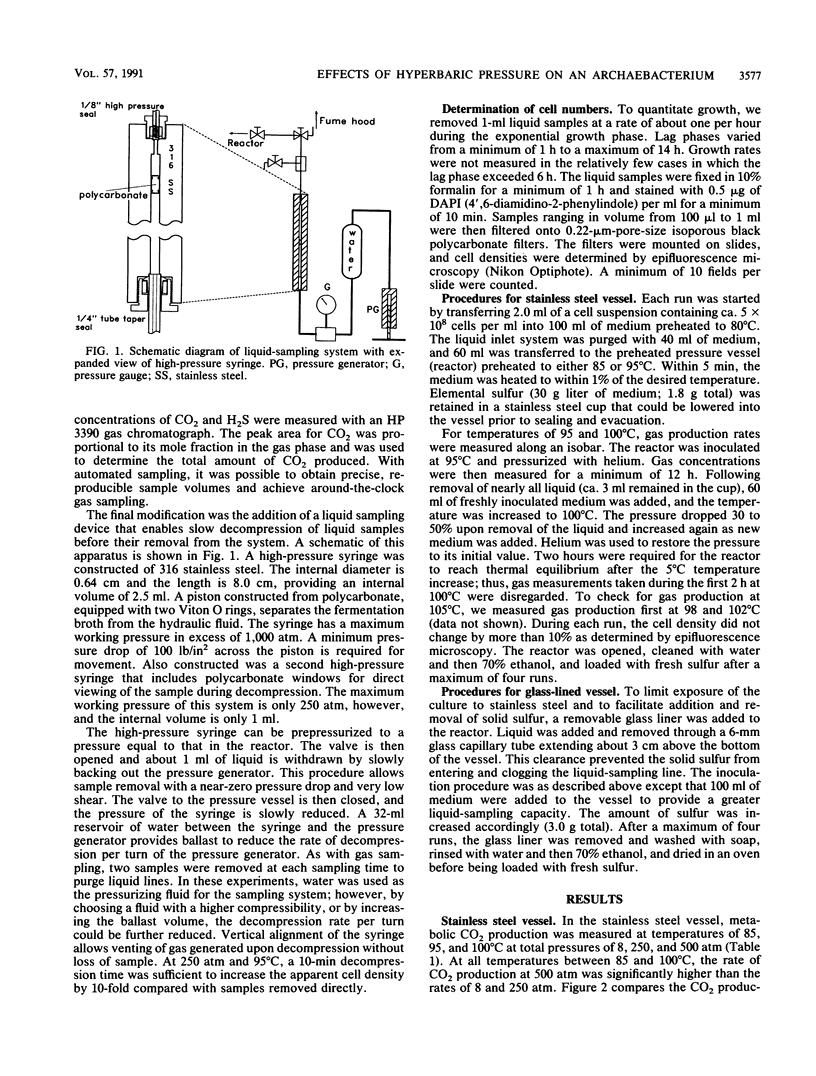
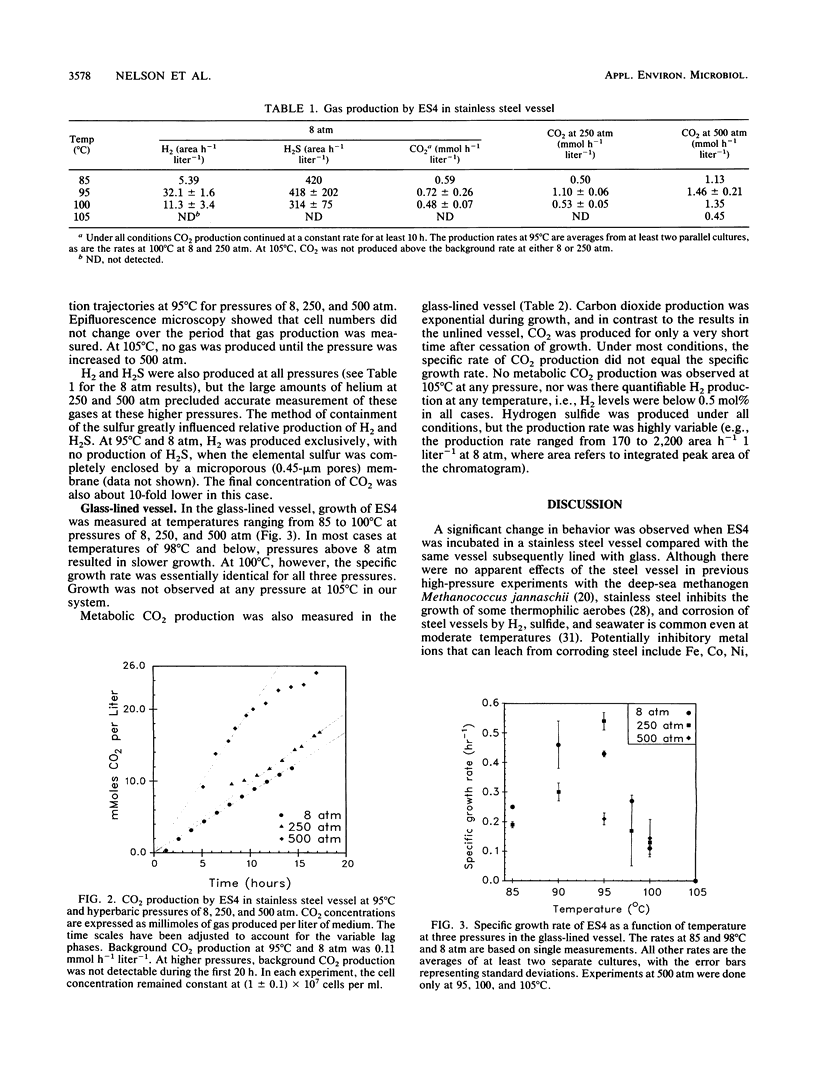
The effects of hyperbaric helium pressures on the growth and metabolism of the deep-sea isolate ES4 were investigated. In a stainless steel reactor, cell growth was completely inhibited but metabolic gas production was observed. From 85 to 100°C, CO2 production proceeded two to three times faster at 500 atm (1 atm = 101.29 kPa) than at 8 atm. At 105°C, no CO2 was produced until the pressure was increased to 500 atm. Hydrogen and H2S were also produced biotically but were not quantifiable at pressures above 8 atm because of the high concentration of helium. In a glass-lined vessel, growth occurred but the growth rate was not accelerated by pressure. In most cases at temperatures below 100°C, the growth rate was lower at elevated pressures; at 100°C, the growth rates at 8, 250, and 500 atm were nearly identical. Unlike in the stainless steel vessel, CO2 production was exponential during growth and continued for only a short time after growth. In addition, relatively little H2 was produced in the glass-lined vessel, and there was no growth or gas production at 105°C at any pressure. The behavior of ES4 as a function of temperature and pressure was thus very sensitive to the experimental conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcuri E. J., Ehrlich H. L. Influence of hydrostatic pressure on the effects of the heavy metal cations of manganese, copper, cobalt, and nickel on the growth of three deep-sea bacterial isolates. Appl Environ Microbiol. 1977 Feb;33(2):282–288. doi: 10.1128/aem.33.2.282-288.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D., Wright M., Yayanos A. A., Silverman M. Isolation of a gene regulated by hydrostatic pressure in a deep-sea bacterium. Nature. 1989 Nov 30;342(6249):572–574. doi: 10.1038/342572a0. [DOI] [PubMed] [Google Scholar]
- Bernhardt G., Jaenicke R., Lüdemann H. D., König H., Stetter K. O. High Pressure Enhances the Growth Rate of the Thermophilic Archaebacterium Methanococcus thermolithotrophicus without Extending Its Temperature Range. Appl Environ Microbiol. 1988 May;54(5):1258–1261. doi: 10.1128/aem.54.5.1258-1261.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Itoh M., Olson G. J., Kelly R. M. Role of Polysulfides in Reduction of Elemental Sulfur by the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 May;56(5):1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer R. W., Hogan P. M., Hugon M., Macdonald A. G., Miller K. W. Patterns of interaction of effects of light metabolically inert gases with those of hydrostatic pressure as such--a review. Undersea Biomed Res. 1982 Dec;9(4):353–396. [PubMed] [Google Scholar]
- Brown S. H., Kelly R. M. Cultivation Techniques for Hyperthermophilic Archaebacteria: Continuous Culture of Pyrococcus furiosus at Temperatures near 100 degrees C. Appl Environ Microbiol. 1989 Aug;55(8):2086–2088. doi: 10.1128/aem.55.8.2086-2088.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain R. A., Yayanos A. A. Ultrastructural changes in an obligately barophilic marine bacterium after decompression. Appl Environ Microbiol. 1991 May;57(5):1489–1497. doi: 10.1128/aem.57.5.1489-1497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss J. B., Dymond J., Gordon L. I., Edmond J. M., von Herzen R. P., Ballard R. D., Green K., Williams D., Bainbridge A., Crane K., van Andel T. H. Submarine thermal sprirngs on the galapagos rift. Science. 1979 Mar 16;203(4385):1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- DeLong E. F., Yayanos A. A. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science. 1985 May 31;228(4703):1101–1103. doi: 10.1126/science.3992247. [DOI] [PubMed] [Google Scholar]
- Deming J. W., Baross J. A. Solid Medium for Culturing Black Smoker Bacteria at Temperatures to 120 degrees C. Appl Environ Microbiol. 1986 Feb;51(2):238–243. doi: 10.1128/aem.51.2.238-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R., Bernhardt G., Lüdemann H. D., Stetter K. O. Pressure-Induced Alterations in the Protein Pattern of the Thermophilic Archaebacterium Methanococcus thermolithotrophicus. Appl Environ Microbiol. 1988 Oct;54(10):2375–2380. doi: 10.1128/aem.54.10.2375-2380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Enzymes under extremes of physical conditions. Annu Rev Biophys Bioeng. 1981;10:1–67. doi: 10.1146/annurev.bb.10.060181.000245. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O., Molyneaux S. J., Langworthy T. A. Extremely thermophilic fermentative archaebacteria of the genus desulfurococcus from deep-sea hydrothermal vents. Appl Environ Microbiol. 1988 May;54(5):1203–1209. doi: 10.1128/aem.54.5.1203-1209.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau J. V. Induction, transcription and translation in Escherichia coli: a hydrostatic pressure study. Biochim Biophys Acta. 1967 Dec 19;149(2):506–512. doi: 10.1016/0005-2787(67)90178-5. [DOI] [PubMed] [Google Scholar]
- MacNaughtan W., Macdonald A. G. Effects of pressure and pressure antagonists on the growth and membrane-bound ATP-ase of Acholeplasma laidlawii B. Comp Biochem Physiol A Comp Physiol. 1982;72(2):405–414. doi: 10.1016/0300-9629(82)90238-9. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Shah N. N., Nelson C. M., Ludlow J. M., Clark D. S. Pressure and Temperature Effects on Growth and Methane Production of the Extreme Thermophile Methanococcus jannaschii. Appl Environ Microbiol. 1988 Dec;54(12):3039–3042. doi: 10.1128/aem.54.12.3039-3042.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morild E. The theory of pressure effects on enzymes. Adv Protein Chem. 1981;34:93–166. doi: 10.1016/s0065-3233(08)60519-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Usui M., Yatome C., Idaka E. Influence of chromium compounds on microbial growth and nucleic acid synthesis. Bull Environ Contam Toxicol. 1989 Aug;43(2):254–260. doi: 10.1007/BF01701756. [DOI] [PubMed] [Google Scholar]
- Reysenbach A. L., Deming J. W. Effects of hydrostatic pressure on growth of hyperthermophilic archaebacteria from the juan de fuca ridge. Appl Environ Microbiol. 1991 Apr;57(4):1271–1274. doi: 10.1128/aem.57.4.1271-1274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Yayanos A. A., Colwell R. R. Metabolic activities of the intestinal microflora of a deep-sea invertebrate. Appl Environ Microbiol. 1976 Jan;31(1):46–48. doi: 10.1128/aem.31.1.46-48.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. Cobalt-induced inhibition of growth in cyanobacteria Anabaena doliolum and Anacystis nidulans: interaction with sulphur containing amino acids. Indian J Exp Biol. 1989 Dec;27(12):1092–1093. [PubMed] [Google Scholar]
- Taylor C. D. Growth of a bacterium under a high-pressure oxy-helium atmosphere. Appl Environ Microbiol. 1979 Jan;37(1):42–49. doi: 10.1128/aem.37.1.42-49.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S. R., Marquis R. E. Microbial growth modification by compressed gases and hydrostatic pressure. Appl Environ Microbiol. 1984 Apr;47(4):780–787. doi: 10.1128/aem.47.4.780-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9542–9546. doi: 10.1073/pnas.83.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOBELL C. E., OPPENHEIMER C. H. Some effects of hydrostatic pressure on the multiplication and morphology of marine bacteria. J Bacteriol. 1950 Dec;60(6):771–781. doi: 10.1128/jb.60.6.771-781.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]


