Abstract
Research reports on de novo neurogenesis, particularly dopaminergic (DA) neurogenesis in the adult mammalian substantia nigra (SN) remain very controversial. For this reason, we utilized the nestin second-intron enhancer controlled LacZ reporter (pNes-LacZ) transgenic mouse model coupled with MPTP lesion system to investigate whether there are neurogenesis and DA neurogenesis in the SN of the adult normal and Parkinson’s disease (PD)–like mice. First, we demonstrated the presence of neural progenitor cells (NPCs), basal levels of neurogenesis, and DA neurogenesis in the normal adult mouse SN. Second, we showed there is not only a significant increase in the number of NPCs, but also a dramatic increase of neurogenesis from the NPCs in the SN and the midline region adjacent to the SN of the PD-like mice, compared with that of normal controls. More importantly, we also demonstrated there is an increase of DA neurogenesis in the SN of the MPTP lesioned mice. Third, we showed that the increased DA neurogenesis in the MPTP lesioned mice was derived from the NPCs and BrdU positive cells, suggesting multiple stem cell lineages may contribute to the enhanced neurogenesis in the adult SN. Taken together, these results establish that there are basal levels, albeit low, and increased levels of de novo neurogenesis and DA neurogenesis in the SN of the adult normal and PD-like mice respectively. The increased NPCs in the MPTP lesioned mice further suggest that experimental approaches to promote de novo neurogenesis may provide an effective therapy for PD by functional replacement of degenerated DAs.
Introduction
It has been well-established that neurogenesis occurs in the hippocampus (1) and olfactory bulb (2,3) of the adult mammalian brain. More recently, neurogenesis has been detected in other anatomic regions of the adult CNS, such as, neocortex (4), amygdala(5-7), striatum (8–12) and spinal cord (13), although some of the reports have not been completely confirmed. In addition, increased neurogenesis has been shown in the animal models of ischemic stroke (14), amyotrophic lateral sclerosis (ALS) (15,16), Alzheimer’s disease (AD) (17), Parkinson’s disease (PD) and epilepsy (18,19). Moreover, enhanced neurogenesis has been reported in human patients with AD (20) and Huntington’s disease (21,22). These results together demonstrate that there is neurogenesis in the normal adult CNS and elevated neurogenic responses in the degenerative CNS. The increased neural progenitor cells (NPCs) and enhanced neurogenesis in human patients with neurodegenerative complications, and in the animal models mimicking human degenerative diseases suggest that measures to promote de novo neurogenesis may have significant therapeutic potential.
Although substantial progress has been made in identifying and characterizing neuronal regeneration in the adult CNS, the research reports on neurogenesis, particularly dopaminergic (DA) neurogenesis in the adult mammalian substantia nigra (SN) remain very controversial. More recently, Zhao et al. (23) reported that dopaminergic neurons (DAs), the cell type lost in PD, were continuously generated in normal adult mice and were enhanced in the PD-like adult mice SN. Moreover, these newly generated DAs can project to the striatum and integrate into synaptic circuits for potential function (23). On the other hand, Frielingsdorf et al., (24) showed no evidence of DA regeneration, and even no neurogenesis in the adult mammalian SN either in the normal or chemical-induced PD-like rodent models using similar experimental procedures. To address the different observations, and more importantly, to identify if there is de novo neurogenesis and DA neurogenesis in the adult SN, we applied the well-established nestin second-intron enhancer controlled LacZ reporter (pNes-LacZ) transgenic mouse model to identify NPCs and analyze their differentiation in the SN with neuron-specific and DA neuron-specific markers. We established that there are NPCs in the adult SN. More importantly, we showed that there are basal levels, albeit low, and increased levels of neurogenesis and DA neurogenesis in the SN of the adult normal and PD-like mice respectively. Therefore, these results suggest that experimental approaches that facilitate de novo neurogenesis, particularly DA neurogenesis may be potentially used for effective therapy of PD.
Materials and Methods
Transgenic mice
Nestin is a marker for neural progenitor cells (NPCs) in the mammalian CNS (25). Nestin second-intron enhancer controlled LacZ reporter (pNes-LacZ) transgenic mice (Jackson Laboratory, Bar Harbor, ME) (26,27) were used to identify NPCs and characterize neurogenesis and DA neurogenesis with specific neuronal markers. All experimental protocols were approved by the Institutional Animal Use and Care Committee and are in agreement with the National Institutes of Health guideline for the care and use of laboratory animals.
1-Methyl-4-Phyenyl-1,2,3,6-Tetrahydropyridine (MPTP) lesion on substantia nigra in adult mice
Transgenic mice (pNes-LacZ) at 60–80 days of age were used for the vehicle control and MPTP lesion. Based on previous MPTP-induction mouse models (28–30), a daily dose of MPTP (Sigma, St. Louis, MO) at 20 mg/kg was administered intraperitoneally (i.p.) to the pNes-LacZ transgenic mice to generate subchronic dopaminergic neuron degeneration mimicking Parkinson’s disease (PD). Vehicle control and MPTP lesioned pNes-LacZ transgenic mice were deeply anesthetized with 50 mg/kg of pentobarbital at the end of experiments. After perfusion with 4% paraforaldehyde (PFA), the mouse brain was dissected out and processed for immunohistochemical (IHC) analysis as described previously (16).
In vivo 5-bromodeoxyuridine (BrdU) labeling
BrdU (Sigma, St. Louis, MO) at 50 mg/kg/day was i.p. administered for up to 15 days to adult pNes-Tg mice (16,31). The mouse brain was dissected out 1 day after the last injection of BrdU and processed for BrdU detection and IHC analysis as described in the following section.
LacZ staining, immunohistochemical staining, image analysis and quantification
The SN region was used to identify NPCs, and to analyze neurogenesis and DA neurogenesis. For LacZ staining, sections (12 μm) were incubated in 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) solution for 16 h at room temperature as previously described (16). For IHC staining, sections were incubated in blocking buffer (10% goat serum/0.2% triton X-100 in 1×PBS, pH 7.5) for 1 h at room temperature. Primary antibody (All from Chemicon International Inc.: anti-TH at 1:300 dilution; anti-NeuN at 1:400 dilution; and anti-BrdU at 1:400 dilution) was then added to the blocking buffer and the section was incubated at 4 °C overnight. The next day, sections were washed 5 times (5 min each) in 1×PBS (pH 7.5) containing 0.5% triton X-100, followed by incubation with specific fluorescein-conjugated secondary antibody (All from Molecular Probe at 1:2000 dilution) for 2 h at room temperature. After extensive washes, sections were covered with anti-fade medium and sealed for fluorescent microscopic analysis. For negative control staining, sections were incubated without primary antibody.
All images were collected and analyzed with a Nikon fluorescent microscope 80I equipped with the Spot digital camera and Photoshop software. Confocal microscope (Zeiss LSM510) was used to confirm the colocalization of double labeling.
Quantification and statistical analysis
Quantifications of LacZ positive alone, LacZ and NeuN positive, LacZ and TH positive, BrdU and TH positive cells in the SN were manually counted every 10th-section between Bregma −2.70 and −3.80 of the mouse brain stereotaxic coordinates under fluorescent microscope. This method of quantification for LacZ (NPCs) analysis eliminates the potential false positive effect that may be caused by the background. The number of cells from bilateral SN were added together and averaged for 3 mice. All the LacZ and NeuN positive, LacZ and TH positive, BrdU and TH positive cells in the SN or the midline region adjacent to SN were confirmed with a Zeiss LSM510 confocal microscope. Statistical analysis of the MPTP lesioned mice compared with that of vehicle controls was performed using the paired Student t test. P < 0.05 was considered significant.
Results
There are neural progenitor cells (NPCs) and basal levels of neurogenesis in the adult mouse SN
Previous reports on the neurogenesis and DA neurogenesis in the adult mammalian SN provided conflicting results (23, 24). For this reason, we utilized the well-defined transgenic mouse model, the nestin second-intron enhancer controlled LacZ reporter (pNes-LacZ) transgenic mice, to identify NPCs in the adult mouse SN and to further analyze the statues of neurogenesis and DA neurogenesis with specific neuronal and DA neuronal markers. Similar to previous finding (23), we did not find significant changes in the number of TH-positive cells in the SN for animal 50 to 180 days of age (data not shown). Thus, we utilized 60–80 days of age mice for the studies of neurogenesis and DA neurogenesis in the adult mouse SN in the current report. With the pNes-LacZ transgenic mouse model, we demonstrated that there are NPCs in the adult mouse SN as identified by LacZ reporter staining (Fig. 1A and 1B), although the number of NPCs in the SN is much less than that of hippocampus (Fig. 1C and 1D) and olfactory bulb (data not shown). In addition, we also detected that there are basal levels of neurogenesis from the NPCs in the adult mouse SN as measured by LacZ and NeuN double staining (Fig. 1A and 1B). Further analysis with confocal microscope confirmed that NeuN staining and LacZ staining are colocalized in the same cells (Fig. 1B insert). However, we did not detect DA neurogenesis from the NPCs in the normal adult mouse SN (n=3 normal control mice ranging from 60 to 80 days of age).
Figure 1. There are NPCs and basal levels of neurogenesis in the normal adult mouse substantia nigra (SN).
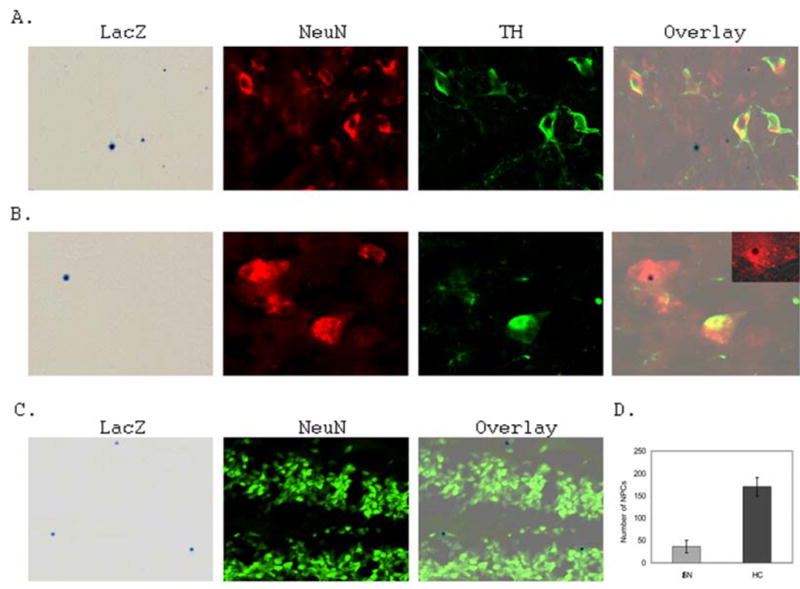
A. Representative fluorescent microscopic images of the normal mouse SN stained with LacZ (Blue), anti-NeuN (Red) and anti-TH (Green) antibodies to identify NPCs, neurons and DAs respectively. Notably, some NPCs are colocalized with NeuN, but not TH (Overlay). B. Representative fluorescent microscopic images of NPCs colocalized with NeuN, confirmed with confocal microscopic analysis (Insert). C. Representative fluorescent microscopic images of the adult normal mouse hippocampus dentate gyrus stained with LacZ (Blue), anti-NeuN (Green) antibody to identify NPCs and neurons respectively. D. Average number of NPCs in the normal adult mouse SN and hippocampus (HC) (n=4 mice).
MPTP lesion depletes the number of dopaminergic neurons (DAs) and increases neurogenesis in the SN
Since the basal levels of neurogenesis are low, and there is no DA neurogenesis detected from the NPCs in the normal adult mouse SN, we then asked whether DA degeneration in Parkinson’s disease (PD) can promote substantial neurogenic and/or DA neurogenic responses. To this end, we adopted the well-established sub-chronic MPTP lesion system to generate DA degeneration mimicking PD in the pNes-LacZ transgenic mice (28–30). MPTP lesion depleted the number of tyrosine hydroxylase (TH) positive DAs (Fig. 2A), and increased the number of NPCs in the SN compared with that of vehicle controls (Fig. 2A and Fig. 2B). More importantly, some of the NPCs in the SN differentiated into neuron-like cells that are positive in NeuN staining (Fig. 2C). Further confocal analysis confirmed that the LacZ staining and NeuN staining are colocalized in the same cells (Fig. 2C insert), demonstrating an increased neurogenesis from the adult NPCs. The quantification of neuron-like NPCs in the SN of the MPTP-induced and vehicle control mice was shown in Fig. 2D.
Figure 2. MPTP lesion depletes TH-positive DAs, increases NPCs and neurogenesis in the adult mouse SN.
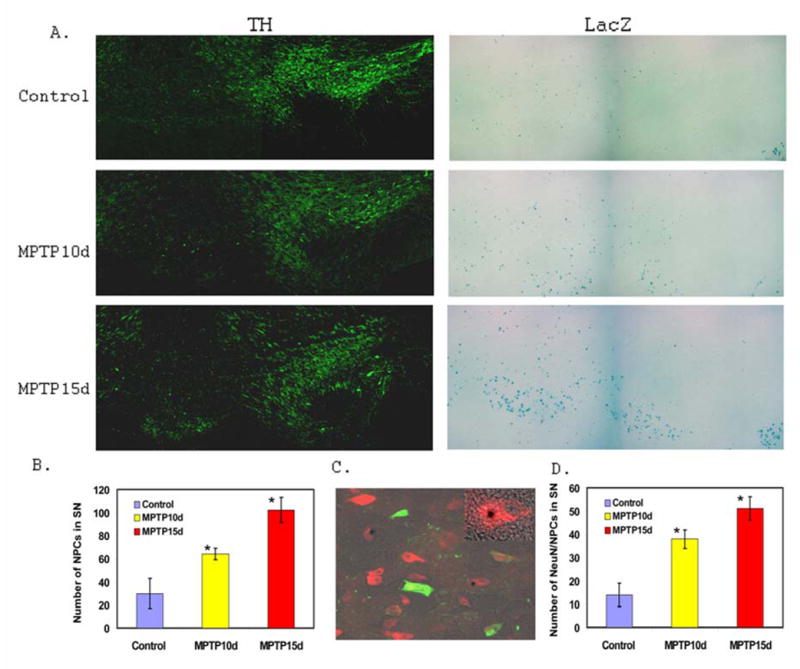
A. Representative fluorescent microscopic images of MPTP lesioned mice demonstrating MPTP depletes TH-positive DAs and increases the number of NPCs in the SN. B. Quantification of NPCs in the SN of vehicle control mice (Control: n=3), MPTP lesioned mice at 10 days (MPTP10d: n=3; *p<0.05 compared with control), and MPTP lesioned mice at 15 days (MPTP15d: n=3; *p<0.05 compared with control). C. Representative fluorescent microscopic images of NPCs colocalized with NeuN in the MPTP lesioned SN, confirmed with confocal microscopic analysis (Insert). D. Quantification of neurogenesis from NPCs in the SN of vehicle control mice (Control: n=3), MPTP lesioned mice at 10 days (MPTP10d: n=3; *p<0.05 compared with control), and MPTP lesioned mice at 15 days (MPTP15d: n=3; *p<0.05 compared with control).
To test if the increased number of NPCs in the SN is from proliferation in situ, we carried out BrdU labeling for up to 15 days of i.p. injection. The number of BrdU-positive cells in the SN (Fig. 3A and Fig. 3B) and midline vicinity to the SN (Fig. 3C and Fig. 3D) was increased in the MPTP lesioned mice (Fig. 3B and Fig. 3D) compared to that of control mice (Fig. 3A and Fig. 3C). However, most of the NPCs did not colocalize with BrdU-positive cells in the SN (Fig. 3A and Fig. 3B), suggesting that the increased NPCs are not derived from the proliferation in situ. Thus, the increased NPCs in the SN after MPTP lesion may result from migration. On the other hand, the percentage of NPCs colocalized with BrdU in the midline vicinity region (adjacent to the SN) was increased (Fig. 3C and Fig. 3D). Further confocal analysis confirmed the colocalization of LacZ and BrdU (Fig. 3E and Fig. 3F).
Figure 3. MPTP lesion increases BrdU-positive cells in the adult mouse SN and adjacent midline region to the SN.
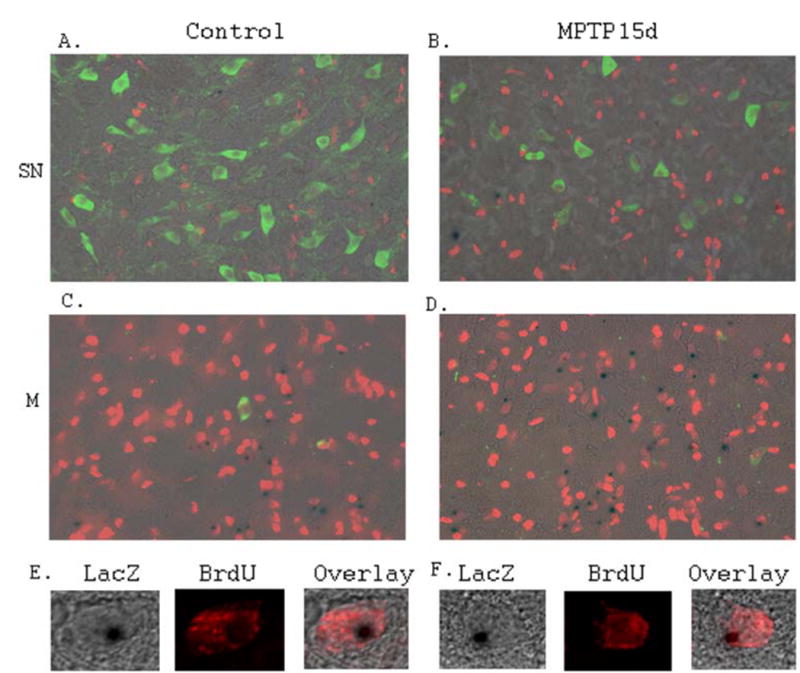
A.Representative fluorescent microscopic image of the control mouse SN stained with LacZ (Blue), anti-BrdU (Red) and anti-TH (Green) antibodies. B. Representative fluorescent microscopic image of the MPTP lesioned mouse SN stained with LacZ (Blue), anti-BrdU (Red) and anti-TH (Green) antibodies. Most of the NPCs in the SN do not colocalize with BrdU. C. Representative fluorescent microscopic image of the control mouse midline region adjacent to the SN, stained with LacZ (Blue), anti-BrdU (Red) and anti-TH (Green) antibodies. Some of the NPCs in the midline region colocalize with BrdU. D. Representative fluorescent microscopic image of the MPTP lesioned mouse midline region adjacent to the SN, stained with LacZ (Blue), anti-BrdU (Red) and anti-TH (Green) antibodies. Some of the NPCs in midline region adjacent to the SN colocalize with BrdU. Notably, there are more NPCs and BrdU positive cells detected in the midline region adjacent to the SN of the MPTP lesioned mice compared to that of controls. E. Representative confocal microscopic images showing LacZ staining and anti-BrdU staining are colocalized in the same cells in the midline region adjacent to the SN of the control mice. F. Representative confocal microscopic images showing LacZ staining and anti-BrdU staining are colocalized in the same cells in the midline region adjacent to the SN in the MPTP lesioned mice.
In addition to the increased NPCs and neurogenesis in the SN (Fig. 2), we also observed a dramatic increase in the number of NPCs in the midline regions adjacent to the TH positive DAs in the SN of the MPTP lesioned mice (Fig. 4B), in comparison with that of vehicle controls (Fig. 4A). More significantly, some of the NPCs in the controls and MPTP lesioned mice were positive for NeuN staining (Fig. 4A–4C). Further confocal analysis showed that NeuN staining and LacZ staining are in the same cells (Fig. 4A and 4B inserts). The quantification of NPCs and NeuN positive NPCs in the midline region adjacent to the SN was shown in Fig. 4C. These NPCs were apparently in the process of migrating toward the SN based on the analysis of MPTP-induced time dependent lesion (data not shown).
Figure 4. MPTP lesion increases neurogenesis in the adult mouse midline region adjacent to the SN.
A. Representative fluorescent microscopic images of the control mouse midline region adjacent to the SN stained with LacZ (Blue), anti-NeuN (Red) antibody, showing some of the LacZ positive cells are NeuN-positive. Confocal analysis confirmed the colocalization of LacZ and NeuN (Insert). B. Representative fluorescent microscopic images of the MPTP lesioned mouse midline region adjacent to the SN stained with LacZ (Blue), anti-NeuN (Red) antibody, showing some of the LacZ positive cells are NeuN-positive. Confocal analysis confirmed the colocalization of LacZ and NeuN (Insert). C. Quantification of NPCs and neurogenesis from NPCs in the midline region adjacent to the SN in the vehicle control and MPTP lesioned mice (Control: n=3; MPTP10d: n=3; MPTP15d: n=3; *p<0.05 compared with control).
MPTP lesion promotes dopaminergic (DA) neurogenesis from the NPCs and the BrdU positive cells in the adult mouse SN
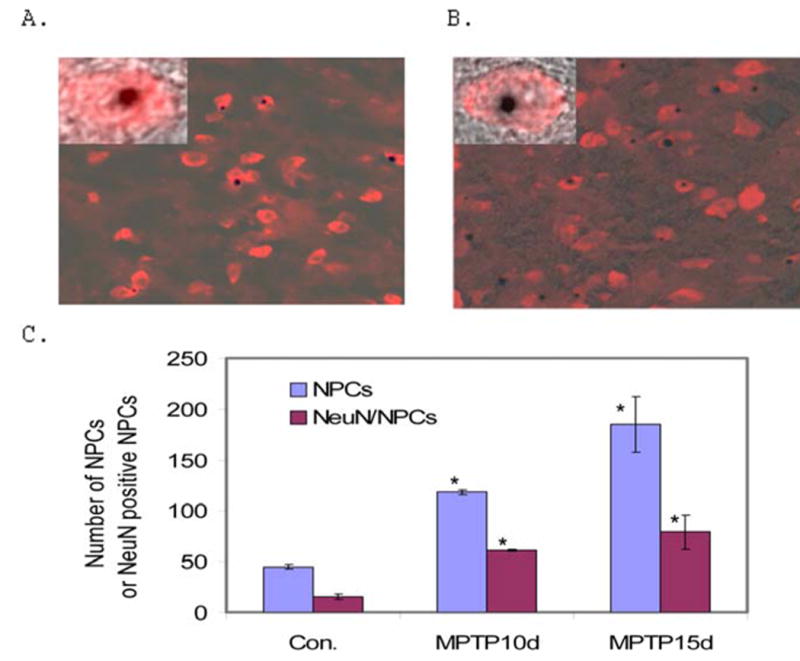
The increased neurogenesis in the SN and the midline region adjacent to the SN promoted us to analyze if there is enhanced DA neurogenesis as a result of MPTP lesion. LacZ staining and TH labeling demonstrated that some NPCs in the SN are TH-positive (Fig. 5A). Further analysis with confocal microscope confirmed the LacZ staining and TH labeling are in the same cell (Fig. 5A Inserts), indicating that there is DA neurogenesis in the SN of MPTP lesioned mice. The quantification of DA neurogenesis from NPCs in the adult mouse SN (from Bregma −2.70 to −3.80 of the mouse brain stereotaxic coordinates) was shown in Fig. 5B (n=4 for control mice; n=3 for MPTP10d mice; n=3 MPTP15d mice).
Figure 5. MPTP lesion increases DA neurogenesis in the adult mouse SN.
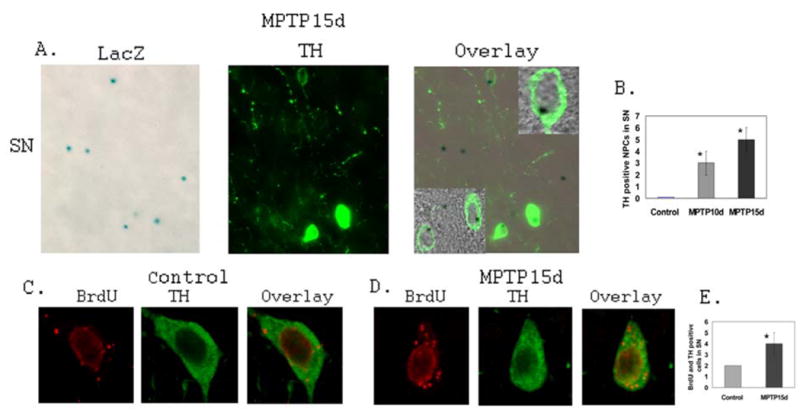
A. Representative fluorescent microscopic images of the vehicle control mouse SN stained with LacZ (Blue) and anti-TH (Green) antibody to identify DA neurogenesis from NPCs. Confocal analysis confirms the LacZ staining and TH staining are colocalized in the same cells (Inserts). B. Quantification of DA neurogenesis from NPCs in the SN of vehicle control and MPTP lesioned mice (Control: n=3; MPTP10d: n=3; MPTP15d: n=3; *p<0.05 compared with control). C. Representative confocal microscopic images showing the colocalization of anti-BrdU staining (Red) and anti-TH (Green) in the same cell in the vehicle control mouse SN. D. Representative confocal microscopic images showing the colocalization of anti-BrdU staining (Red) and anti-TH (Green) in the same cell in the MPTP lesioned mouse SN. E. Quantification of DA neurogenesis from BrdU-positive cells in the SN of vehicle control and MPTP lesioned mice (Control: n=3; MPTP15d: n=3; *p<0.05 compared with control).
Similarly, we also demonstrated that there is an increase of DA neurogenesis from some of the BrdU positive cells in the SN of MPTP lesioned mice (Fig. 5C and Fig.5D). Double (BrdU and TH) labeling and quantitative analyses showed there is a significant increase of the DA neurogenesis from BrdU-positive cells in the MPTP lesioned mice compared with that of vehicle controls (Fig. 5E) (n=4 for control mice; n=3 MPTP15d mice). These data together demonstrate that MPTP-mediated DA degeneration promotes DA neurogenesis in the SN.
Discussion
Although much evidence has demonstrated that neurogenesis is present in the adult mammalian CNS, the status of neurogenesis, particularly DA neurogenesis in the SN has not been unambiguously defined. Most of the previous studies relied on the colocalization of BrdU labeling and specific neuronal marker(s), and/or DA marker(s) to define neurogenesis and/or DA neurogenesis in the adult SN (1, 22, 23). On the other hand, neurogenesis and/or DA neurogenesis could result from the non-proliferative NPCs, which are present in the adult CNS (16). For this reason, we chose the pNes-LacZ transgenic mice to study neurogenesis and DA neurogenesis in the SN. We wished to use this mouse model to clarify the controversial data currently existed in the area. More importantly, we wished to identify if there are NPCs in the adult SN and to analyze their status of neurogenesis and DA neurogenesis, particularly under the MPTP lesion conditions. Selection of the pNes-LacZ transgenic mouse model for this study was primarily based on two major characteristics: 1). The pNes-LacZ transgenic mice carry LacZ reporter gene under the control of the nestin second-intron enhancer, which allows us to identify NPCs in the adult CNS (16, 27); 2). By coupling LacZ staining with BrdU labeling and specific neuron identification, we can define the proliferation and differentiation status of the BrdU-positive cells and NPCs in the SN. The present study using the pNes-LacZ transgenic mice demonstrates three major findings: 1). There are basal levels of neurogenesis and DA neurogenesis detected in the normal adult mouse SN; 2). MPTP lesion depleting DAs promotes neurogenesis and DA neurogensis in the adult mouse SN; 3). MPTP lesion-induced DA neurogenesis may be derived from multiple stem lineages including the NPCs and BrdU labeled proliferative cells. Taken together, this study provides compelling evidence supporting the presence of the basal levels of neurogenesis in the normal adult mouse SN, and enhanced DA neurogenesis in the MPTP lesioned mice mimicking PD.
Our findings with the pNes-LacZ transgenic mice using both BrdU labeling and NPC identification approaches establish that there is de novo neurogenesis and DA neurogenesis in the adult SN. However, the rate of basal levels of neurogenesis and DA neurogensis is low in the normal adult mouse SN as compared with that of hippocampus and olfactory bulb (Fig. 1; Fig. 2) (23). In fact, we did not detect DA neurogenesis from the NPCs, even though there are NPCs and there is neurogenesis in the normal adult mouse SN (Fig. 1 and Fig. 2). On the other hand, we identified and confirmed that a few BrdU positive cells are immunostained with TH in the SN (Fig. 5C and Fig. 5E), indicating there are basal levels of DA neurogenesis. These data are, in some respects, in agreement with those reported by Zhao et al. (23), who demonstrated there is DA neurogenesis in the SN. Nevertheless, our observations, although support some of Zhao et al.’s findings, are different from those reported by Frielingsdorf et al, (24), who suggest there is no DA neurogenesis, and even suggest there is no neurogenesis in the adult SN either under normal or PD-like lesioned conditions. Though Zhao et al. (23) and Frielingsdorf et al., (24) applied similar animal models and experimental procedures to identify neurogenesis, their reports on neurogenesis and/or DA neurogenesis in the adult SN remain discrepant. The different observations are unlikely to be from the different animal models they used, but may be due to the BrdU dose, section thickness, and parameters used to analyze neurogenesis (23, 24). It should be pointed out that the neuronal (DA) differentiation from BrdU positive (neural) stem cells may represent a portion of neurogenesis (16). The labeling approach by retroviral vector carrying reporter gene coupled with specific neuronal marker(s) may identify the same group(s) of neurogenic cells as that of the method with BrdU (23, 24). On the other hand, neurogenesis may derive from pre-existed progenitor cells which are at an inert state and are not proliferative. Thus, in our current studies, we focused on the pNes-LacZ transgenic mouse model and paid special attention to the above-mentioned parameters to analyze neurogenesis and DA neurogenesis. For the BrdU experiments, we administered BrdU i.p. at a dose 50mg/kg daily for up to15 days with the stock solution at 10 mg/ml. The same or similar BrdU labeling conditions have been used to identify stem (neural progenitor) cell types in CNS (16; 17; 30). For the IHC staining, we utilized the sections at 12 μm thickness, which allows the antibody to thoroughly penetrate to the cellular and subcellular structures. For double labeling and detection, all images were initially analyzed with fluorescent microscope, and subsequently confirmed with confocal microscope. More importantly, we analyzed the neurogenesis and DA neurogenesis from NPCs and BrdU positive cells. With these identification approaches, we demonstrated there are basal levels of neurogenesis in the adult SN (Fig. 1A, 1B and 1D; Fig. 2D). In addition, we showed there is DA neurogenesis from the BrdU-positive cells (Fig.5), although we did not detect DA neurogenesis from the NPCs in the normal adult SN (Fig. 1A and Fig. 5B).
After MPTP lesion, there was an increase of NPCs (Fig. 2) and BrdU positive cells (Fig. 3) in the SN. More significantly, there was enhanced neurogenesis and DA neurogenesis detected from NPCs, and BrdU positive cells (Fig. 5). The reports from Zhao et al. (23), Frielingsdorf et al. (24), and previous studies (32,33) utilized BrdU labeling coupled with specific neuronal marker(s) as a major approach to identify DA neurogenesis in the SN. While it is true to claim there is DA neurogenesis in the SN, if BrdU is colocalized with TH (23). However, it may not be true to claim there is no DA neurogenesis, if BrdU labeling is not colocalized with TH staining, because the DA neurogenesis may result from other progenitor (precursor) cells, for example, NPCs, which are in a non-proliferative state. Because the higher dose of BrdU may label apoptotic and atrophic cells (24, 34), we further determined if neurogenesis and DA neurogenesis can be derived from the NPCs. As shown in Fig. 1, Fig. 2 and Fig. 5, we demonstrated that LacZ defined NPCs colocalize with not only NeuN staining, but also TH-staining, suggesting there is enhanced neurogenesis and DA neurogenesis in the SN upon MPTP lesion. It should be pointed out that even though there is an increase of DA neurogenesis in MPTP lesioned SN, the relative neurogenic rate and specific numbers of TH-positive-LacZ positive and TH-positive-BrdU-positive DA-like neurons are low (Fig. 2; Fig. 5; and Table 1 of Zhao et al., ). Because of the topology, we found it is very difficulty to accurately count the number of NPCs, BrdU-positive cells, LacZ-positive/TH-positive, and BrdU-positive/TH positive cells in the whole SN. For this reason, we made consecutive sections, and counted from every 10th section between Bregma −2.70 and Bregma −3.80 of the mouse brain stereotaxic coordinates, which cover the major part of mouse SN. With the unbiased and non-overlapped counting approach, we demonstrated that there are about 2 newly generated DAs in the adult mouse SN during the 15 days of BrdU labeling period. Upon MPTP lesion, we showed that there are about 9 (5 from NPCs and 4 from BrdU positive cells) newly generated DAs in the mouse SN during the same period of time. Using the same cell counting method, we demonstrated that there are more NPCs (Fig. 2) and BrdU cells (Fig. 3A and 3B) in the SN of MPTP lesioned mice compared to controls. Because BrdU labeling with the daily dose of 50 mg/kg for 15 days during the MPTP lesion did not label NPCs in the SN (Fig. 3A and Fig. 3B), we think the NPCs present in the region are not proliferative. More significantly, NPCs and neurogenic BrdU-positive cells may represent different stem cell sources, some of which differentiate toward DAs after MPTP lesion. In fact, a few NPCs and BrdU positive cells were demonstrated to colocalize with TH-positive DAs respectively in the MPTP lesioned mice, indicating DA neurogenesis in the adult mouse SN may be derived from multiple cell lineages (Fig. 5). More recently, data from Yoshimi et al. (35) suggest a possibility of neurogenesis in SN of Parkinsonian brain. The large number of NPCs and BrdU positive cells and low DA neurogenesis rate in the MPTP lesioned SN further provide a rational for therapy of DA degeneration in PD by stimulation of de novo neurogenesis from both NPCs and BrdU-positive cells.
One important, but not unambiguously resolved issue in previous and current studies is where the increased NPCs come from in the MPTP lesioned mice. Based on the distribution pattern, colocalization of LacZ staining and BrdU labeling, we think that the increased NPCs in the SN are derived from the subventricular zone (SVZ). Though most of the NPCs distributed in the SN do not label with BrdU, the LacZ positive NPCs in the ventricular systems, particularly in the ependymal layer, were stained with BrdU, indicating these NPCs are proliferative (data not shown). Since there are NPCs in the SN, and these NPCs were not proliferative in situ, we reasoned that the increased NPCs in the MPTP lesioned mouse SN are putatively derived from the cerebroventricular system. However, extensive analyses are required to determine and to define the migratory pathways of NPCs from subventricular zone to the SN in response to DA neuron degeneration.
In summary, using the pNes-LacZ transgenic mouse model and MPTP lesion system mimicking PD, we demonstrated that degeneration of DAs increases the number of NPCs, and promotes neurogenesis and DA neurogenesis in the adult SN compared with that of normal controls. The newly generated DAs after MPTP lesion apparently result from different stem cell lineages. The increased NPCs, neurogenesis and DA neurogenesis in the PD-like mice suggest that experimental approaches that enhance de novo neurogenesis may contribute significantly to the effective therapy of PD.
Acknowledgments
This study was partially supported by National Institutes of Health Grants NS45829, HL75034 and AG23923, and MDA grant.
Reference List
- 1.van PH, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber PC. Neurogenesis and regeneration in the primary olfactory pathway of mammals. Bibl Anat. 1982:12–25. [PubMed] [Google Scholar]
- 3.Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 5.Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- 6.Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin T, Arvidsson A, Kokaia Z, Lindvall O. Quantitative analysis of the generation of different striatal neuronal subtypes in the adult brain following excitotoxic injury. Exp Neurol. 2005 doi: 10.1016/j.expneurol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Ferri AL, Cavallaro M, Braida D, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 10.Sadikot AF, Sasseville R. Neurogenesis in the mammalian neostriatum and nucleus accumbens: parvalbumin-immunoreactive GABAergic interneurons. J Comp Neurol. 1997;389:193–211. [PubMed] [Google Scholar]
- 11.Song DD, Harlan RE. Genesis and migration patterns of neurons forming the patch and matrix compartments of the rat striatum. Brain Res Dev Brain Res. 1994;83:233–245. doi: 10.1016/0165-3806(94)00144-8. [DOI] [PubMed] [Google Scholar]
- 12.Jin K, Sun Y, Xie L, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 13.Chernoff EA, Sato K, Corn A, Karcavich RE. Spinal cord regeneration: intrinsic properties and emerging mechanisms. Semin Cell Dev Biol. 2002;13:361–368. doi: 10.1016/s1084952102000927. [DOI] [PubMed] [Google Scholar]
- 14.Jin K, Minami M, Xie L, et al. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 15.Klein SM, Behrstock S, McHugh J, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 16.Chi L, Ke Y, Luo C, et al. Motor Neuron Degeneration Promotes Neural Progenitor Cell Proliferation, Migration and Neurogenesis in the Spinal Cords of ALS Mice. Stem Cells. 2005 doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin K, Galvan V, Xie L, et al. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci U S A. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crespel A, Rigau V, Coubes P, et al. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis. 2005;19:436–450. doi: 10.1016/j.nbd.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128:431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RL. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience. 2005;132:777–788. doi: 10.1016/j.neuroscience.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Momma S, Delfani K, et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2004;101:10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuhashi T, Aoki Y, Eksioglu YZ, et al. Overexpression of p27Kip1 lengthens the G1 phase in a mouse model that targets inducible gene expression to central nervous system progenitor cells. Proc Natl Acad Sci U S A. 2001;98:6435–6440. doi: 10.1073/pnas.111051398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki Y, Huang Z, Thomas SS, et al. Increased susceptibility to ischemia-induced brain damage in transgenic mice overexpressing a dominant negative form of SHP2. FASEB J. 2000;14:1965–1973. doi: 10.1096/fj.00-0105com. [DOI] [PubMed] [Google Scholar]
- 28.Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson's disease. J Neural Transm. 2001;108:1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- 30.Bezard E, Dovero S, Bioulac B, Gross C. Effects of different schedules of MPTP administration on dopaminergic neurodegeneration in mice. Exp Neurol. 1997;148:288–292. doi: 10.1006/exnr.1997.6648. [DOI] [PubMed] [Google Scholar]
- 31.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 32.Kay JN, Blum M. Differential response of ventral midbrain and striatal progenitor cells to lesions of the nigrostriatal dopaminergic projection. Dev Neurosci. 2000;22:56–67. doi: 10.1159/000017427. [DOI] [PubMed] [Google Scholar]
- 33.Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Khodor BF, Oo TF, Kholodilov N, Burke RE. Ectopic expression of cell cycle markers in models of induced programmed cell death in dopamine neurons of the rat substantia nigra pars compacta. Exp Neurol. 2003;179:17–27. doi: 10.1006/exnr.2002.8047. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimi K, Ren YR, Seki T, et al. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol. 2005;58:31–40. doi: 10.1002/ana.20506. [DOI] [PubMed] [Google Scholar]


