Abstract
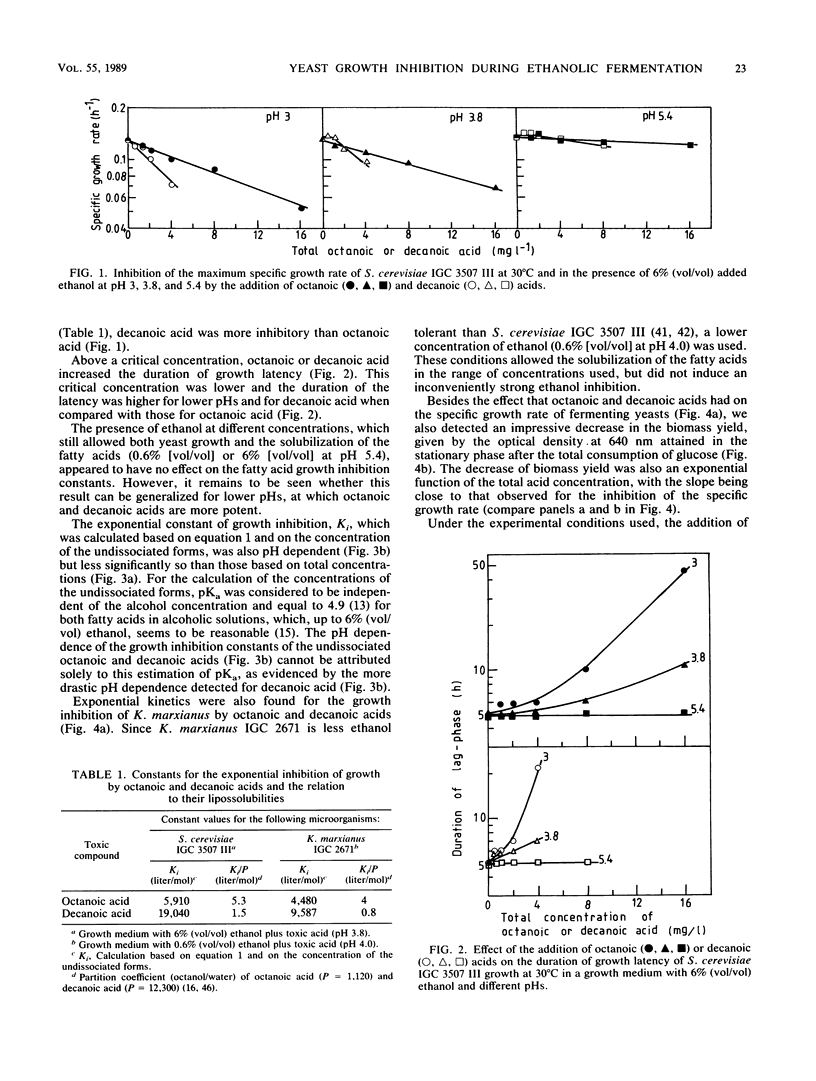
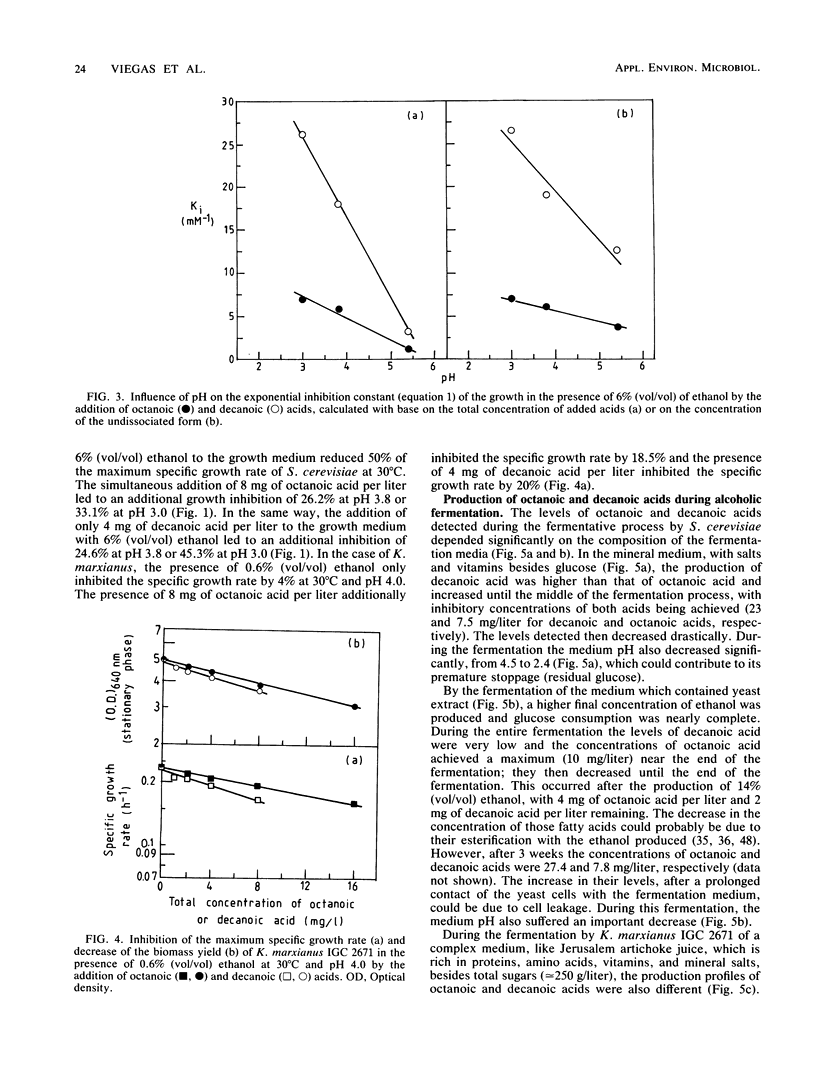
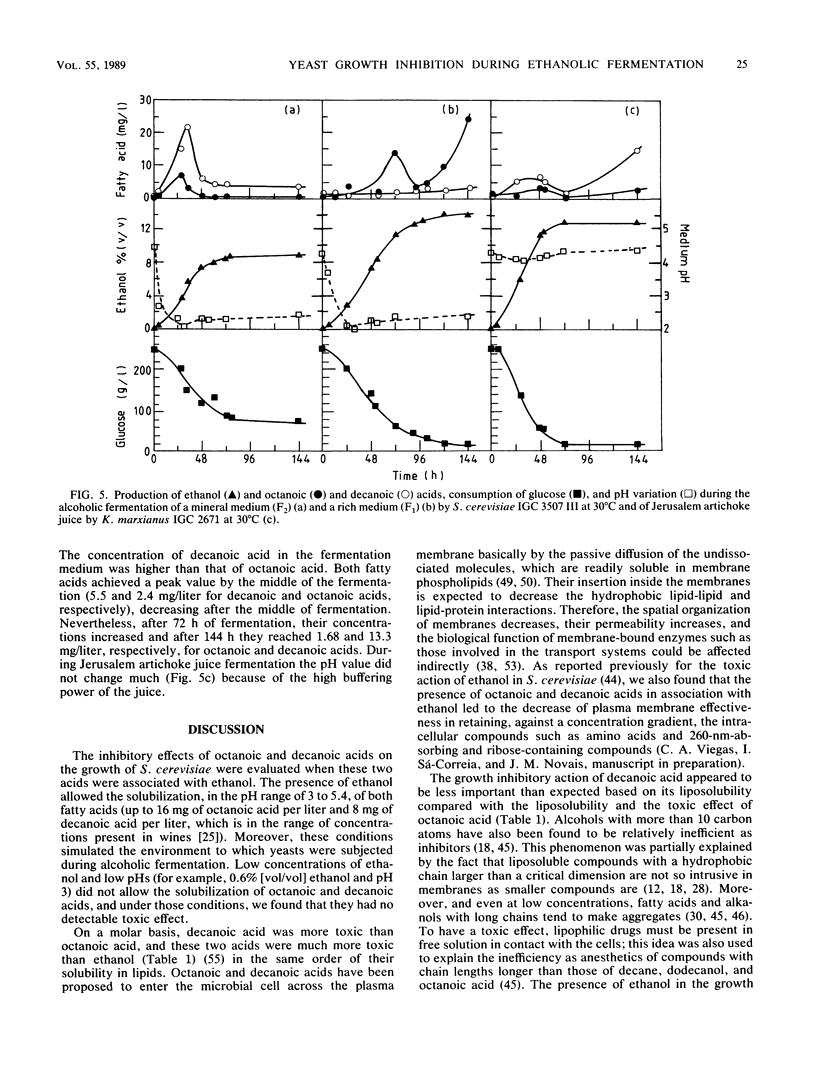
The inhibition of growth by octanoic or decanoic acids, two subproducts of ethanolic fermentation, was evaluated in Saccharomyces cerevisiae and Kluyveromyces marxianus in association with ethanol, the main product of fermentation. In both strains, octanoic and decanoic acids, at concentrations up to 16 and 8 mg/liter, respectively, decreased the maximum specific growth rate and the biomass yield at 30°C as an exponential function of the fatty acid concentration and increased the duration of growth latency. These toxic effects increased with a decrease in pH in the range of 5.4 to 3.0, indicating that the undissociated form is the toxic molecule. Decanoic acid was more toxic than octanoic acid. The concentrations of octanoic and decanoic acids were determined during the ethanolic fermentation (30°C) of two laboratory media (mineral and complex) by S. cerevisiae and of Jerusalem artichoke juice by K. marxianus. Based on the concentrations detected (0.7 to 23 mg/liter) and the kinetics of growth inhibition, the presence of octanoic and decanoic acids cannot be ignored in the evaluation of the overall inhibition of ethanolic fermentation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Bender G. R., Marquis R. E. Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl Environ Microbiol. 1987 Sep;53(9):2124–2128. doi: 10.1128/aem.53.9.2124-2128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. P., Veazey F. J., Rose A. H. Effect of ethanol on activity of the plasma-membrane ATPase in, and accumulation of glycine by, Saccharomyces cerevisiae. J Gen Microbiol. 1987 Apr;133(4):857–865. doi: 10.1099/00221287-133-4-857. [DOI] [PubMed] [Google Scholar]
- Corner T. R. Synergism in the inhibition of Bacillus subtilis by combinations of lipophilic weak acids and fatty alcohols. Antimicrob Agents Chemother. 1981 Jun;19(6):1082–1085. doi: 10.1128/aac.19.6.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O. Ethanol production during batch fermentation with Saccharomyces cerevisiae: changes in glycolytic enzymes and internal pH. Appl Environ Microbiol. 1987 Jun;53(6):1286–1291. doi: 10.1128/aem.53.6.1286-1291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O. Intracellular accumulation of AMP as a cause for the decline in rate of ethanol production by Saccharomyces cerevisiae during batch fermentation. Appl Environ Microbiol. 1988 Jan;54(1):98–104. doi: 10.1128/aem.54.1.98-104.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund T. Inhibition of growth and uptake processes in bacteria by some chemical food preservatives. J Appl Bacteriol. 1980 Jun;48(3):423–432. doi: 10.1111/j.1365-2672.1980.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Eliasz A. W., Chapman D., Ewing D. F. Phospholipid phase transitions. Effects of n-alcohols, n-monocarboxylic acids, phenylalkyl alcohols and quaternary ammonium compounds. Biochim Biophys Acta. 1976 Oct 5;448(2):220–230. doi: 10.1016/0005-2736(76)90238-8. [DOI] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Hansch C., Glave W. R. Structure-activity relationships in membrane-perturbing agents. Hemolytic, narcotic, and antibacterial compounds. Mol Pharmacol. 1971 May;7(3):337–354. [PubMed] [Google Scholar]
- Krebs H. A., Wiggins D., Stubbs M., Sols A., Bedoya F. Studies on the mechanism of the antifungal action of benzoate. Biochem J. 1983 Sep 15;214(3):657–663. doi: 10.1042/bj2140657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon-Lafourcade S., Geneix C., Ribéreau-Gayon P. Inhibition of alcoholic fermentation of grape must by Fatty acids produced by yeasts and their elimination by yeast ghosts. Appl Environ Microbiol. 1984 Jun;47(6):1246–1249. doi: 10.1128/aem.47.6.1246-1249.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G. Interactions between anesthetics and lipid mixtures. Normal alcohols. Biochemistry. 1976 Jun 1;15(11):2448–2454. doi: 10.1021/bi00656a031. [DOI] [PubMed] [Google Scholar]
- Leão C., Van Uden N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jul 11;774(1):43–48. doi: 10.1016/0005-2736(84)90272-4. [DOI] [PubMed] [Google Scholar]
- Pang K. Y., Chang T. L., Miller K. W. On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol Pharmacol. 1979 May;15(3):729–738. [PubMed] [Google Scholar]
- Salgueiro S. P., Sá-Correia I., Novais J. M. Ethanol-Induced Leakage in Saccharomyces cerevisiae: Kinetics and Relationship to Yeast Ethanol Tolerance and Alcohol Fermentation Productivity. Appl Environ Microbiol. 1988 Apr;54(4):903–909. doi: 10.1128/aem.54.4.903-909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sheu C. W., Salomon D., Simmons J. L., Sreevalsan T., Freese E. Inhibitory effects of lipophilic acids and related compounds on bacteria and mammalian cells. Antimicrob Agents Chemother. 1975 Mar;7(3):349–363. doi: 10.1128/aac.7.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Effect of benzoic Acid on growth yield of yeasts differing in their resistance to preservatives. Appl Environ Microbiol. 1988 Aug;54(8):2091–2095. doi: 10.1128/aem.54.8.2091-2095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


