Abstract
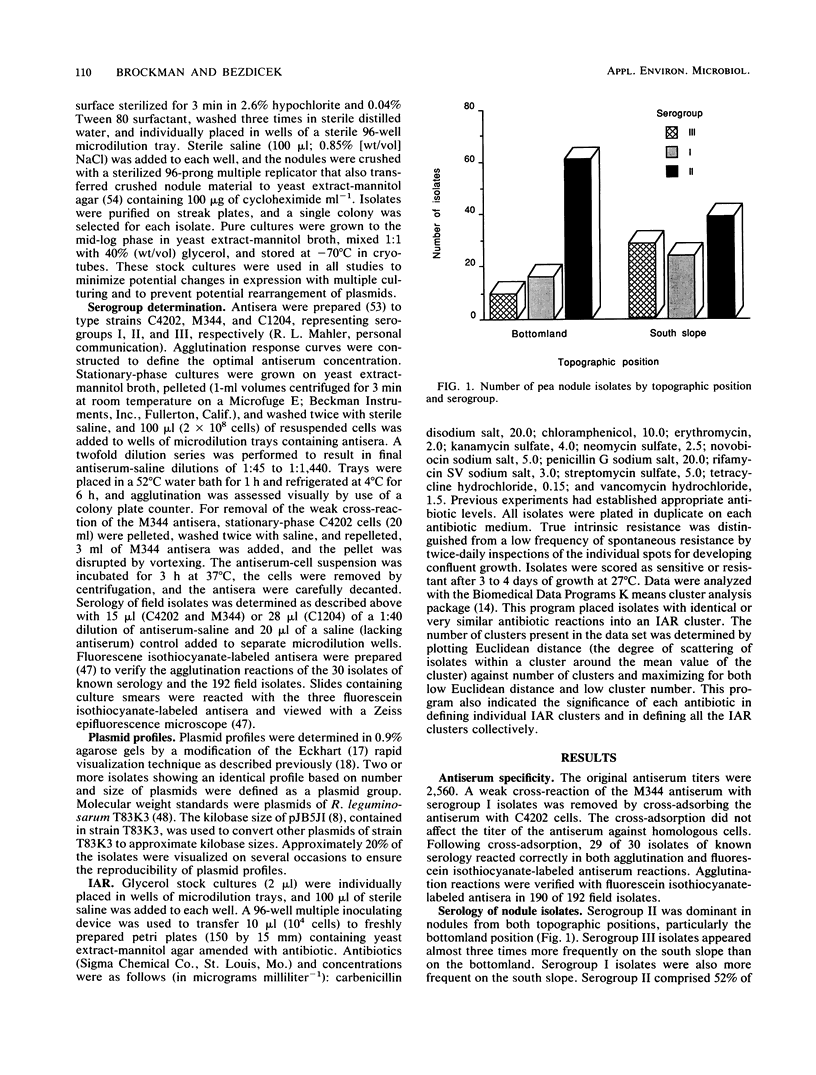
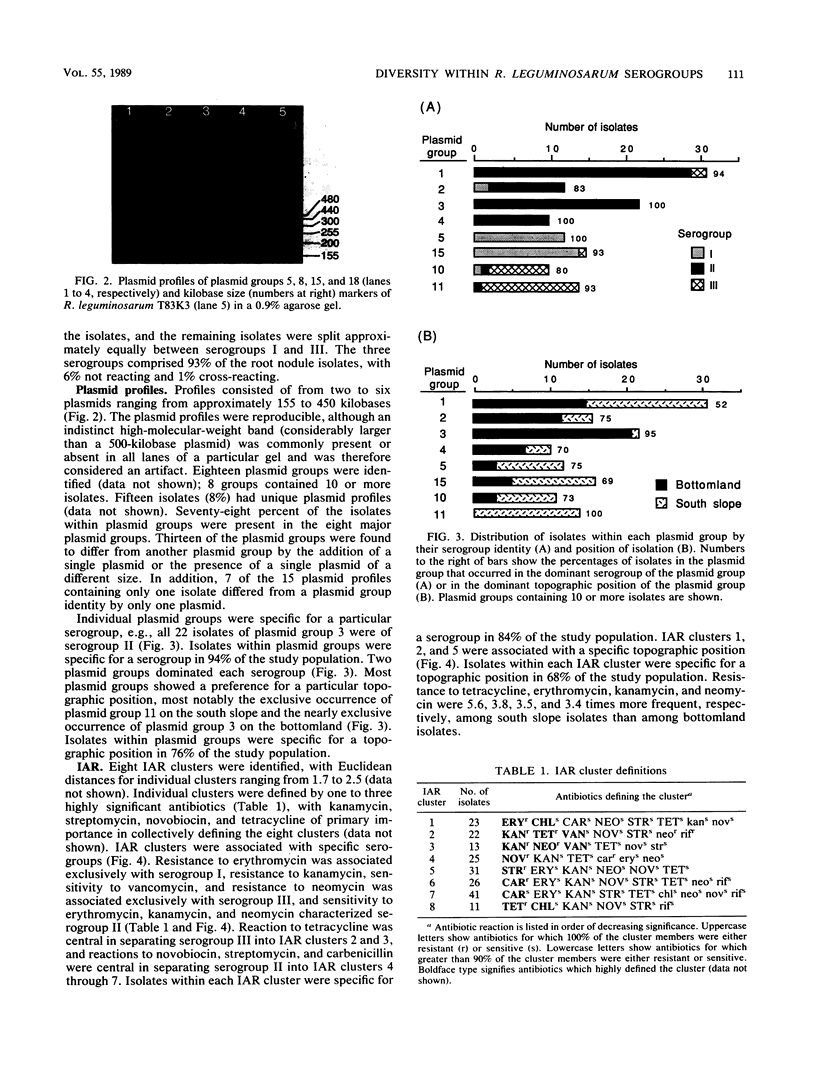
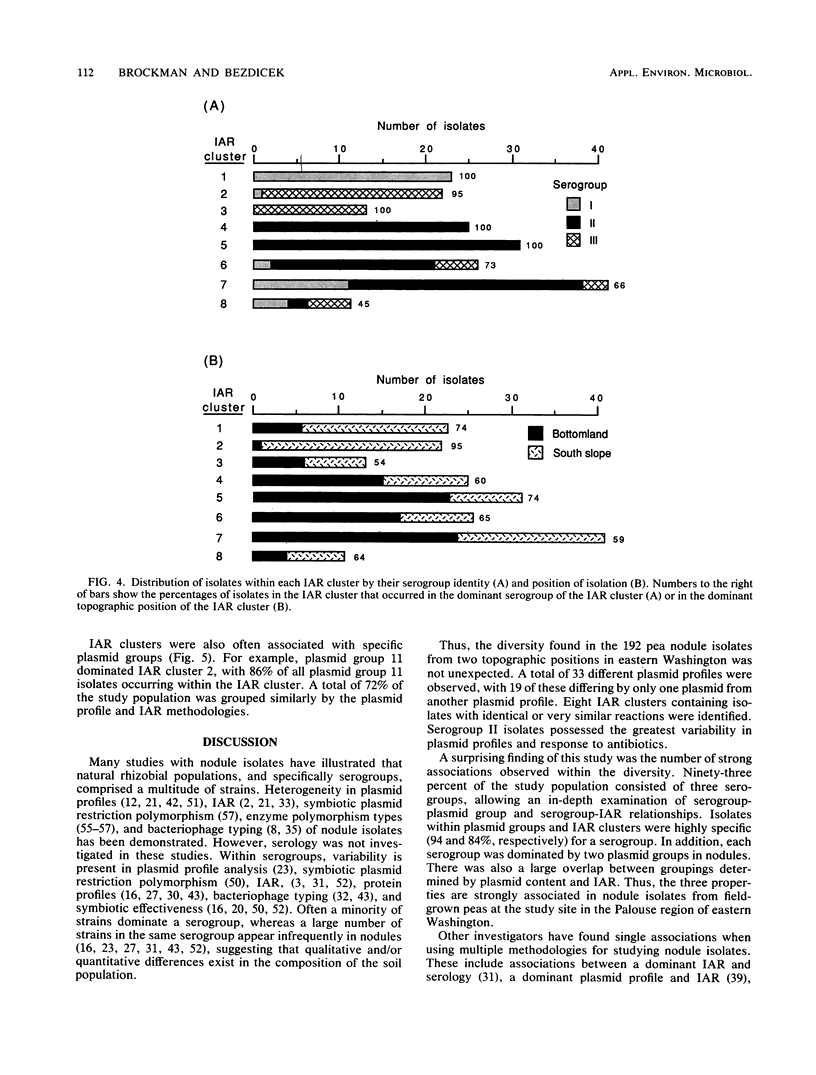
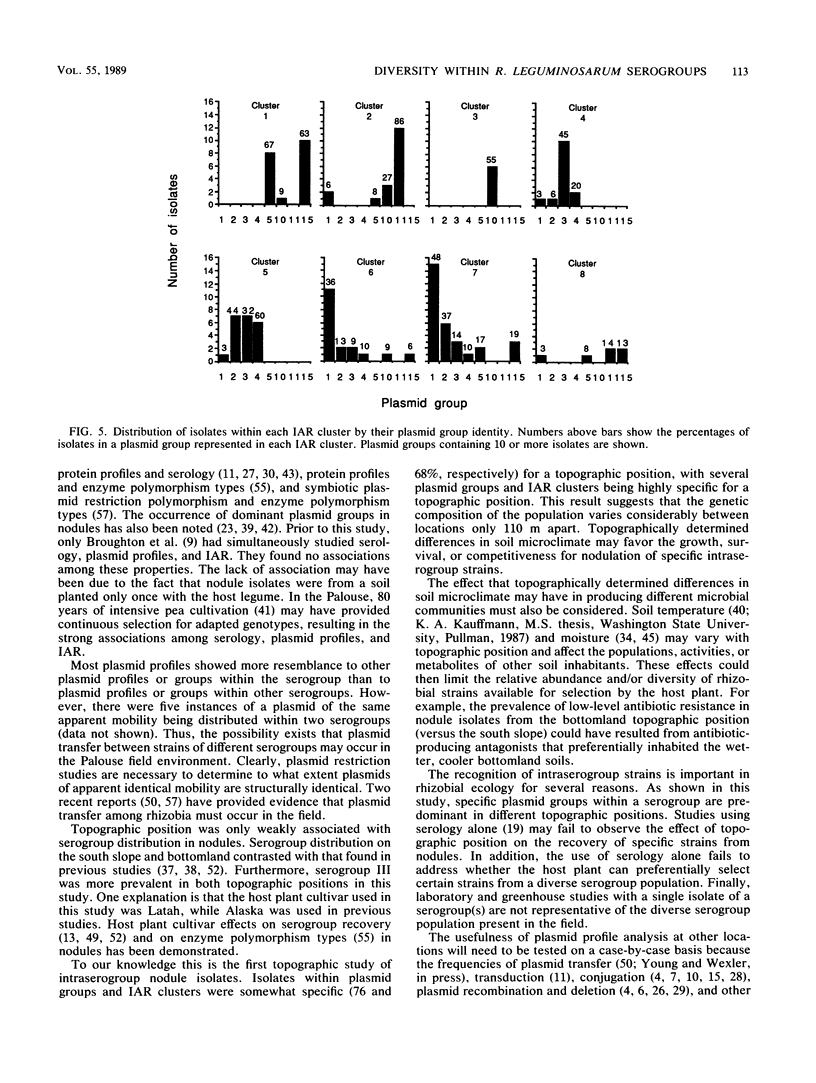
Serology, plasmid profiles, and intrinsic antibiotic resistance (IAR) were determined for 192 isolates of Rhizobium leguminosarum biovar viceae from nodules of peas (Pisum sativum L.) grown on the south slope and bottomland topographic positions in eastern Washington State. A total of 3 serogroups and 18 plasmid profile groups were identified. Nearly all isolates within each plasmid profile group were specific for one of the three serogroups. Cluster analysis of IAR data showed that individual clusters were dominated by one serogroup and by one or two plasmid profile groups. Plasmid profile analysis and IAR analysis grouped 72% of the isolates similarly. Most plasmid profile groups and several IAR clusters favored either the south slope or the bottomland topographic position. These findings show that certain intraserogroup strains possess a greater competitiveness for nodulation and/or possess a greater ability to survive in adjacent soil environments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarger N., Lobreau J. P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982 Sep;44(3):583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromfield E. S., Sinha I. B., Wolynetz M. S. Influence of Location, Host Cultivar, and Inoculation on the Composition of Naturalized Populations of Rhizobium meliloti in Medicago sativa Nodules. Appl Environ Microbiol. 1986 May;51(5):1077–1084. doi: 10.1128/aem.51.5.1077-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Shine J., Rolfe B. G. Sym plasmid transfer to various symbiotic mutants of Rhizobium trifolii, R. leguminosarum, and R. meliloti. J Bacteriol. 1983 Dec;156(3):1035–1045. doi: 10.1128/jb.156.3.1035-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dughri M. H., Bottomley P. J. Effect of Acidity on the Composition of an Indigenous Soil Population of Rhizobium trifolii Found in Nodules of Trifolium subterraneum L. Appl Environ Microbiol. 1983 Nov;46(5):1207–1213. doi: 10.1128/aem.46.5.1207-1213.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson J. K., Bezdicek D. F., Brockman F. J., Li S. W. Enumeration of Tn5 mutant bacteria in soil by using a most- probable-number-DNA hybridization procedure and antibiotic resistance. Appl Environ Microbiol. 1988 Feb;54(2):446–453. doi: 10.1128/aem.54.2.446-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T., Bohlool B. B., Singleton P. W. Bradyrhizobium japonicum-Environment Interactions: Nodulation and Interstrain Competition in Soils along an Elevational Transect. Appl Environ Microbiol. 1987 May;53(5):1113–1117. doi: 10.1128/aem.53.5.1113-1117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer R. J., Peterson H. L. Nodulation efficiency of legume inoculation as determined by intrinsic antibiotic resistance. Appl Environ Microbiol. 1982 Mar;43(3):636–642. doi: 10.1128/aem.43.3.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K. A., Barnet Y. M., McGilchrist C. A. Environmental Factors Influencing Numbers of Rhizobium leguminosarum biovar trifolii and Its Bacteriophages in Two Field Soils. Appl Environ Microbiol. 1987 May;53(5):1125–1131. doi: 10.1128/aem.53.5.1125-1131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler R. L., Bezdicek D. F. Diversity of Rhizobium leguminosarum in the Palouse of Eastern Washington. Appl Environ Microbiol. 1978 Nov;36(5):780–782. doi: 10.1128/aem.36.5.780-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J., Higgins P., O'gara F. Studies on the Inoculation and Competitiveness of a Rhizobium leguminosarum Strain in Soils Containing Indigenous Rhizobia. Appl Environ Microbiol. 1985 Apr;49(4):899–903. doi: 10.1128/aem.49.4.899-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozo T., Cabrera E., Ruiz-Argüeso T. Diversity of Plasmid Profiles and Conservation of Symbiotic Nitrogen Fixation Genes in Newly Isolated Rhizobium Strains Nodulating Sulla (Hedysarum coronarium L.). Appl Environ Microbiol. 1988 May;54(5):1262–1267. doi: 10.1128/aem.54.5.1262-1267.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Gibson A. H., Dudman W. F., Watson J. M. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl Environ Microbiol. 1987 Dec;53(12):2942–2947. doi: 10.1128/aem.53.12.2942-2947.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. P., Demetriou L., Apte R. G. Rhizobium Population Genetics: Enzyme Polymorphism in Rhizobium leguminosarum from Plants and Soil in a Pea Crop. Appl Environ Microbiol. 1987 Feb;53(2):397–402. doi: 10.1128/aem.53.2.397-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



