Abstract
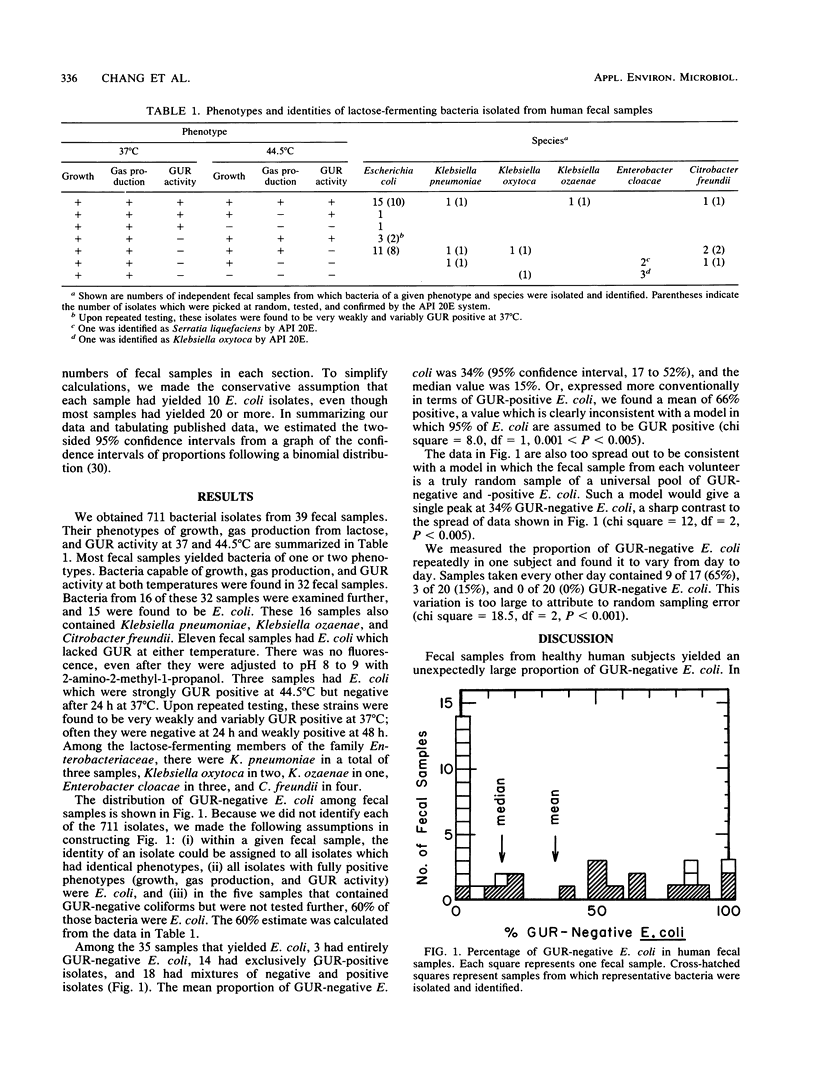
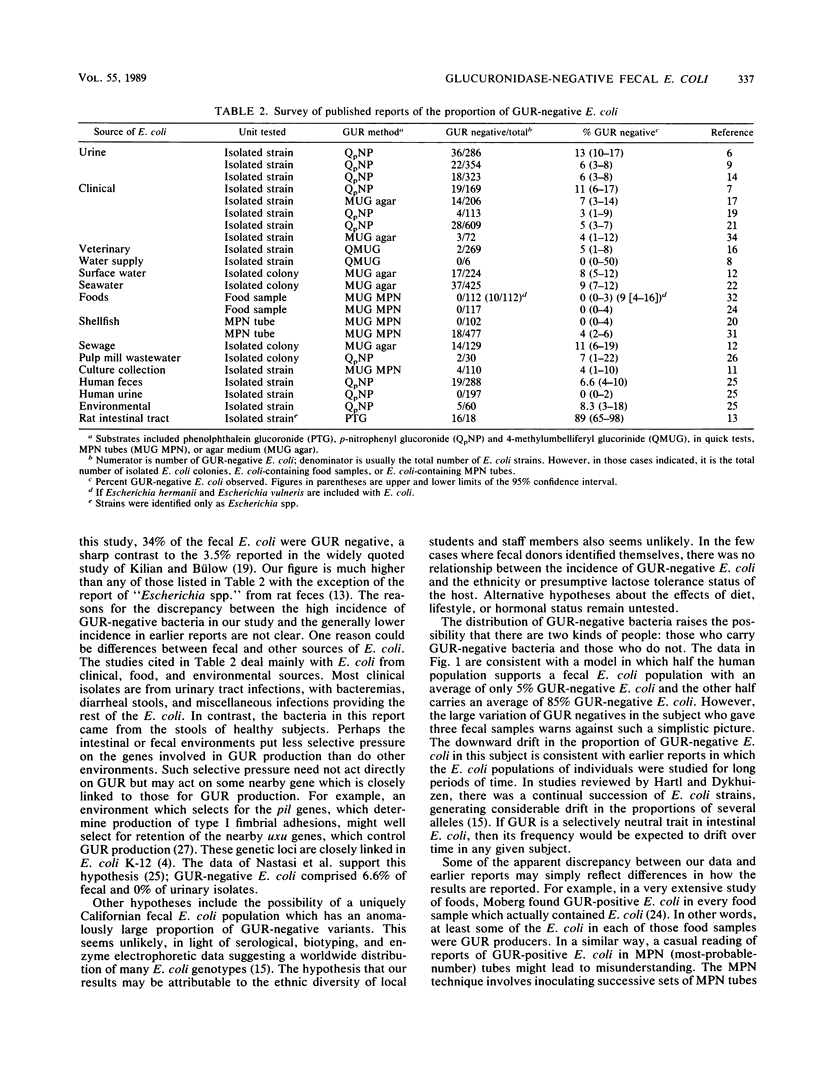
Convenient assays and reports that almost all clinical isolates of Escherichia coli produce beta-D-glucuronidase (GUR) have led to great interest in the use of the enzyme for the rapid detection of the bacterium in water, food, and environmental samples. In these materials, E. coli serves as an indicator of possible fecal contamination. Therefore, it was crucial to examine the proportion of GUR-negative E. coli in human fecal samples. The bacterium was isolated from 35 samples, and a mean of 34% and a median of 15% were found to be GUR negative in lauryl sulfate tryptose broth with 4-methylumbelliferyl-beta-D-glucuronide. E. coli from three samples were temperature dependent for GUR production: very weakly positive at 37 degrees C but strongly positive at 44.5 degrees C. These results remind us of differences between fecal and clinical E. coli populations, of diversity in GUR regulation and expression in natural populations of E. coli, and of the need for caution in using GUR for the detection of fecal E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Dibb W. L., Bottolfsen K. L. Evaluation of Rosco diagnostic beta-glucuronidase tablets in the identification of urinary isolates of Escherichia coli. Acta Pathol Microbiol Immunol Scand B. 1984 Oct;92(5):261–264. doi: 10.1111/j.1699-0463.1984.tb02831.x. [DOI] [PubMed] [Google Scholar]
- Edberg S. C., Kontnick C. M. Comparison of beta-glucuronidase-based substrate systems for identification of Escherichia coli. J Clin Microbiol. 1986 Sep;24(3):368–371. doi: 10.1128/jcm.24.3.368-371.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Piscitelli V., Cartter M. Phenotypic characteristics of coliform and noncoliform bacteria from a public water supply compared with regional and national clinical species. Appl Environ Microbiol. 1986 Sep;52(3):474–478. doi: 10.1128/aem.52.3.474-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Trepeta R. W. Rapid and economical identification and antimicrobial susceptibility test methodology for urinary tract pathogens. J Clin Microbiol. 1983 Dec;18(6):1287–1291. doi: 10.1128/jcm.18.6.1287-1291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P. C., Hartman P. A. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl Environ Microbiol. 1982 Jun;43(6):1320–1329. doi: 10.1128/aem.43.6.1320-1329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier T. A., Hartman P. A. Improved membrane filtration media for enumeration of total coliforms and Escherichia coli from sewage and surface waters. Appl Environ Microbiol. 1987 Jun;53(6):1246–1250. doi: 10.1128/aem.53.6.1246-1250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadelle D., Raibaud P., Sacquet E. beta-Glucuronidase activities of intestinal bacteria determined both in vitro and in vivo in gnotobiotic rats. Appl Environ Microbiol. 1985 Mar;49(3):682–685. doi: 10.1128/aem.49.3.682-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Yourassowsky E. Detection of beta-glucuronidase in lactose-fermenting members of the family Enterobacteriaceae and its presence in bacterial urine cultures. J Clin Microbiol. 1984 Dec;20(6):1177–1179. doi: 10.1128/jcm.20.6.1177-1179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E. The population genetics of Escherichia coli. Annu Rev Genet. 1984;18:31–68. doi: 10.1146/annurev.ge.18.120184.000335. [DOI] [PubMed] [Google Scholar]
- Iritani B., Inzana T. J. Evaluation of a rapid tube assay for presumptive identification of Escherichia coli from veterinary specimens. J Clin Microbiol. 1988 Mar;26(3):564–566. doi: 10.1128/jcm.26.3.564-566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. L., Yeoman P. Detection of specific bacterial enzymes by high contrast metal chelate formation. Part II. Specific detection of Escherichia coli on multipoint-inoculated plates using 8-hydroxyquinoline-beta-D-glucuronide. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):316–321. doi: 10.1016/s0176-6724(88)80047-6. [DOI] [PubMed] [Google Scholar]
- Kaspar C. W., Hartman P. A., Benson A. K. Coagglutination and enzyme capture tests for detection of Escherichia coli beta-galactosidase, beta-glucuronidase, and glutamate decarboxylase. Appl Environ Microbiol. 1987 May;53(5):1073–1077. doi: 10.1128/aem.53.5.1073-1077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Bülow P. Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidases. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):245–251. doi: 10.1111/j.1699-0463.1976.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mates A., Schaffer M. Quantitative determination of Escherichia coli from faecal coliforms in seawater. Microbios. 1988;53(216-217):161–165. [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg L. J. Fluorogenic assay for rapid detection of Escherichia coli in food. Appl Environ Microbiol. 1985 Dec;50(6):1383–1387. doi: 10.1128/aem.50.6.1383-1387.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastasi A., Massenti M. F., Scarlata G. Glucuronidase activity in Escherichia coli. Boll Ist Sieroter Milan. 1982;61(5):441–441. [PubMed] [Google Scholar]
- Niemi R. M., Niemelä S., Mentu J., Siitonen A. Growth of Escherichia coli in a pulp and cardboard mill. Can J Microbiol. 1987 Jun;33(6):541–545. doi: 10.1139/m87-091. [DOI] [PubMed] [Google Scholar]
- Novel M., Novel G. Regulation of beta-glucuronidase synthesis in Escherichia coli K-12: pleiotropic constitutive mutations affecting uxu and uidA expression. J Bacteriol. 1976 Jul;127(1):418–432. doi: 10.1128/jb.127.1.418-432.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez J. L., Berrocal C. I., Berrocal L. Evaluation of a commercial beta-glucuronidase test for the rapid and economical identification of Escherichia coli. J Appl Bacteriol. 1986 Dec;61(6):541–545. doi: 10.1111/j.1365-2672.1986.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Robison B. J. Evaluation of a fluorogenic assay for detection of Escherichia coli in foods. Appl Environ Microbiol. 1984 Aug;48(2):285–288. doi: 10.1128/aem.48.2.285-288.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepeta R. W., Edberg S. C. Methylumbelliferyl-beta-D-glucuronide-based medium for rapid isolation and identification of Escherichia coli. J Clin Microbiol. 1984 Feb;19(2):172–174. doi: 10.1128/jcm.19.2.172-174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]



