Abstract
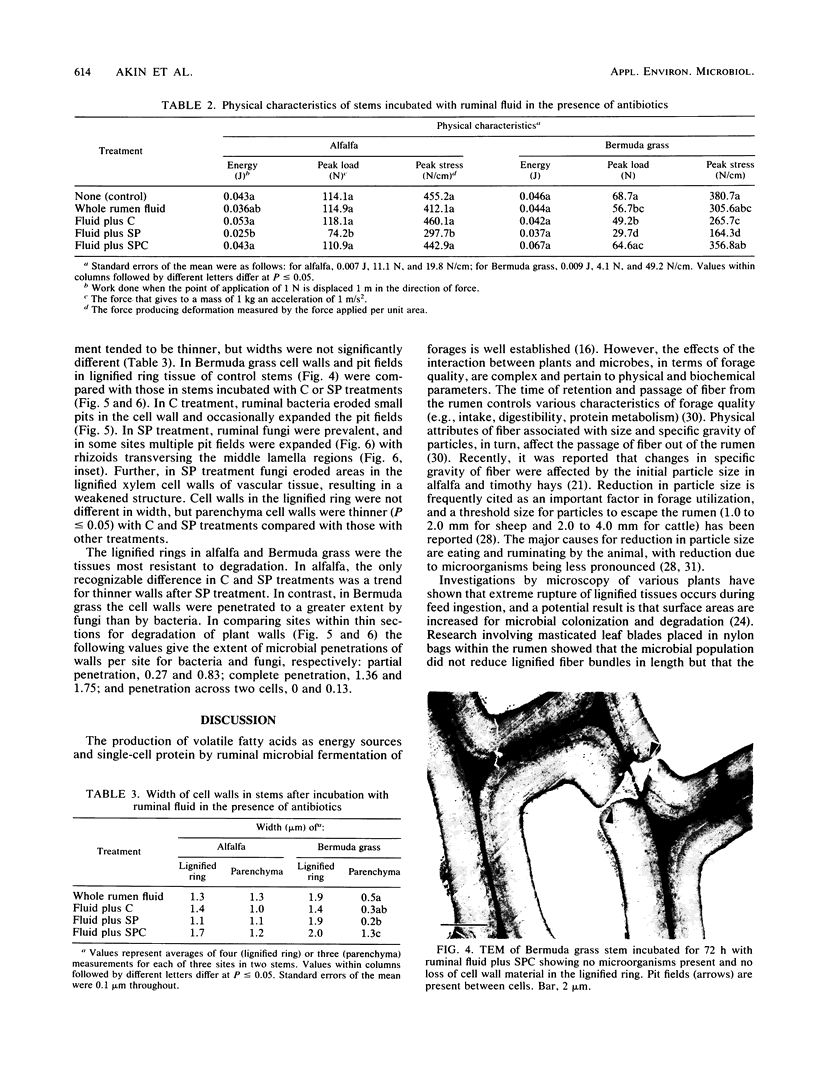
Ruminal bacteria or fungi were selected by the addition of cycloheximide or streptomycin and penicillin, respectively, to ruminal fluid, and the weakening and degradation of lignified tissues in alfalfa and Bermuda grass stems by these treatments and whole ruminal fluid were evaluated in vitro. Dry weight loss in alfalfa was similar for whole ruminal fluid and streptomycin-penicillin treatment, whereas that with streptomycin-penicillin treatment was significantly higher (P ≤ 0.05) than that with cycloheximide treatment. In Bermuda grass, dry weight loss was significantly higher with streptomycin-penicillin than that with whole ruminal fluid and cycloheximide treatment, which were equal. Both peak load (Newtons) and peak stress were less (P ≤ 0.05) for streptomycin-penicillin treatment than with other treatments in both forages. Fungi colonized the lignified ring in alfalfa and tended to reduce the width of cell walls in this tissue, but a large number of fungal penetrations through cell walls was not observed. In contrast, fungal rhizoids frequently penetrated into and through cell walls in the lignified ring of Bermuda grass, often expanding the pit fields between the cells. Ruminal fungi disrupt lignified tissues in stems, and their activity results in a weakened residue more amendable to physical degradation. This weakening may allow plant digesta to be more easily broken apart during animal's rumination and thus facilitate digesta flow and fiber utilization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Benner R. Degradation of polysaccharides and lignin by ruminal bacteria and fungi. Appl Environ Microbiol. 1988 May;54(5):1117–1125. doi: 10.1128/aem.54.5.1117-1125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Rigsby L. L. Mixed fungal populations and lignocellulosic tissue degradation in the bovine rumen. Appl Environ Microbiol. 1987 Sep;53(9):1987–1995. doi: 10.1128/aem.53.9.1987-1995.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Use of chitinase to assess ruminal fungi associated with plant residues in vitro. Appl Environ Microbiol. 1987 Aug;53(8):1955–1958. doi: 10.1128/aem.53.8.1955-1958.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos H. E., Akin D. E. Rumen protozoal degradation of structurally intact forage tissues. Appl Environ Microbiol. 1978 Sep;36(3):513–522. doi: 10.1128/aem.36.3.513-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. The rumen ciliate epidinium in primary degradation of plant tissues. Appl Environ Microbiol. 1979 Jun;37(6):1217–1223. doi: 10.1128/aem.37.6.1217-1223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo M. A., Minson D. J. The effect of pelleting on the voluntary intake and digestibility of leaf and stem fractions of three grasses. Br J Nutr. 1975 Mar;33(2):159–170. doi: 10.1079/bjn19750021. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol. 1987 Jun;53(6):1216–1223. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol. 1975 Dec;91(2):249–262. doi: 10.1099/00221287-91-2-249. [DOI] [PubMed] [Google Scholar]
- Pearce P. D., Bauchop T. Glycosidases of the rumen anaerobic fungus Neocallimastix frontalis grown on cellulosic substrates. Appl Environ Microbiol. 1985 May;49(5):1265–1269. doi: 10.1128/aem.49.5.1265-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M. C., Minson D. J. Fertilizer sulphur as a factor affecting voluntary intake, digestibility and retention time of pangola grass (Digitaria decumbens) by sheep. Br J Nutr. 1978 Jan;39(1):5–11. doi: 10.1079/bjn19780005. [DOI] [PubMed] [Google Scholar]
- Welch J. G. Physical parameters of fiber affecting passage from the rumen. J Dairy Sci. 1986 Oct;69(10):2750–2754. doi: 10.3168/jds.S0022-0302(86)80723-8. [DOI] [PubMed] [Google Scholar]