Abstract
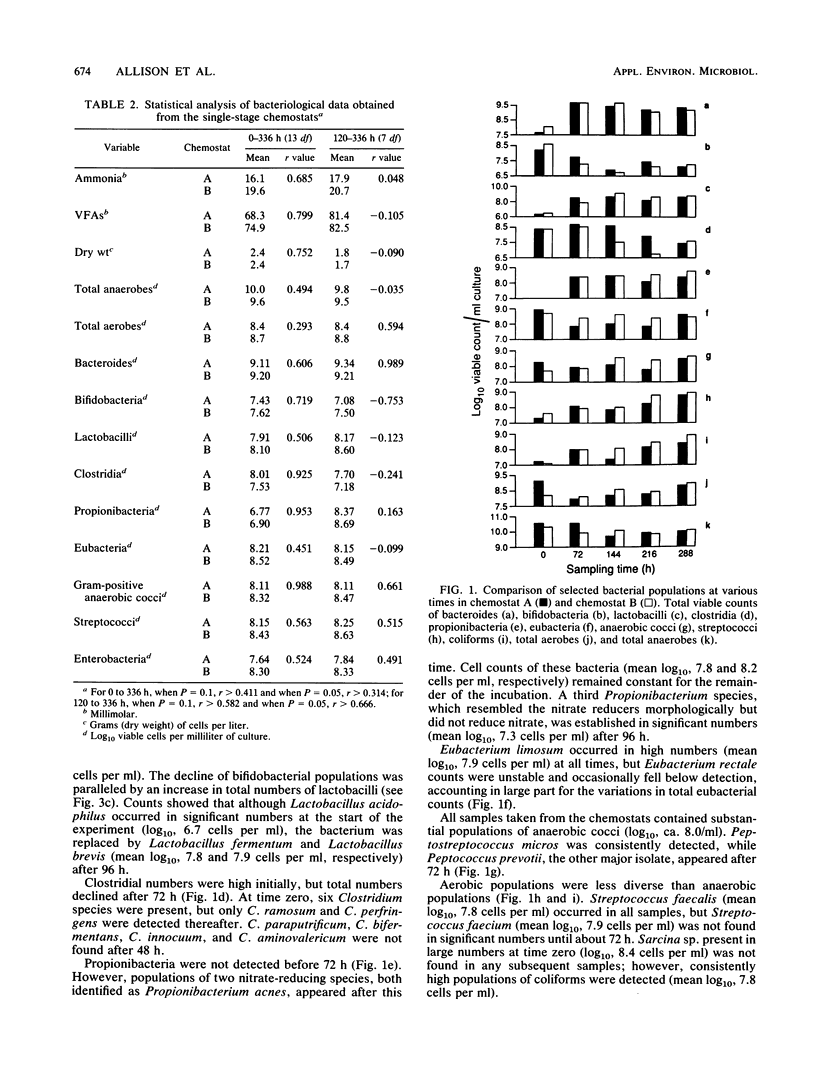
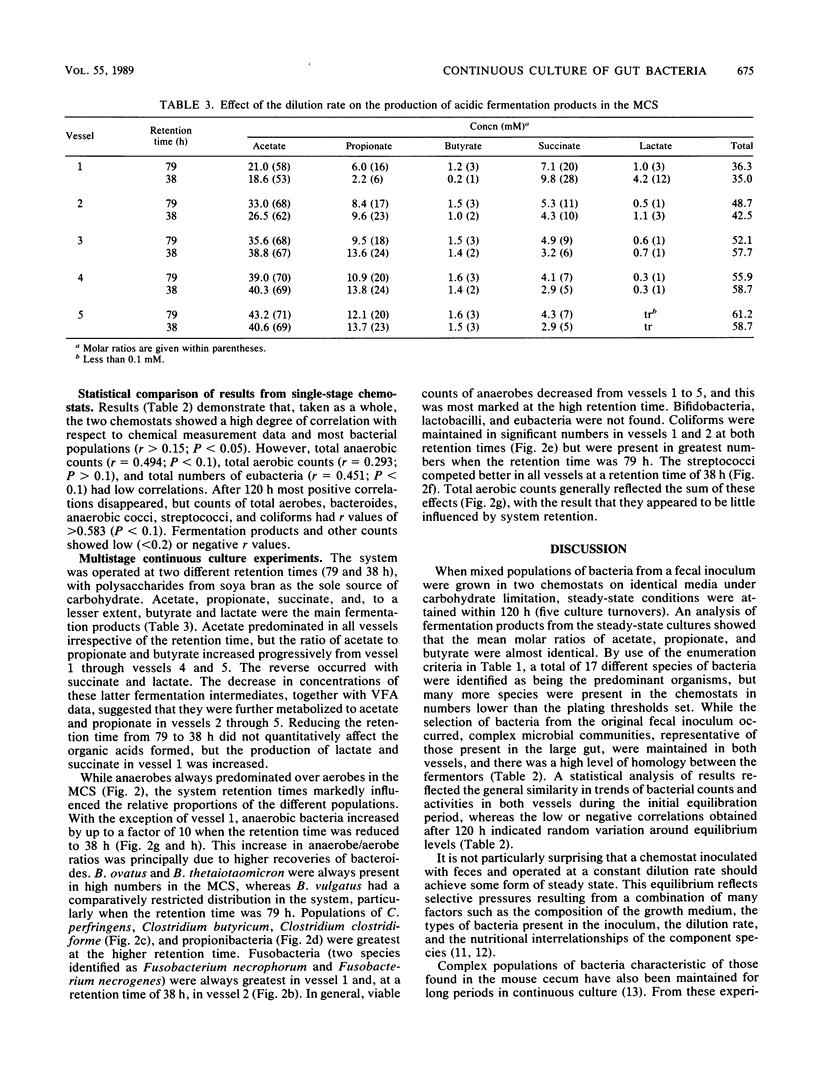
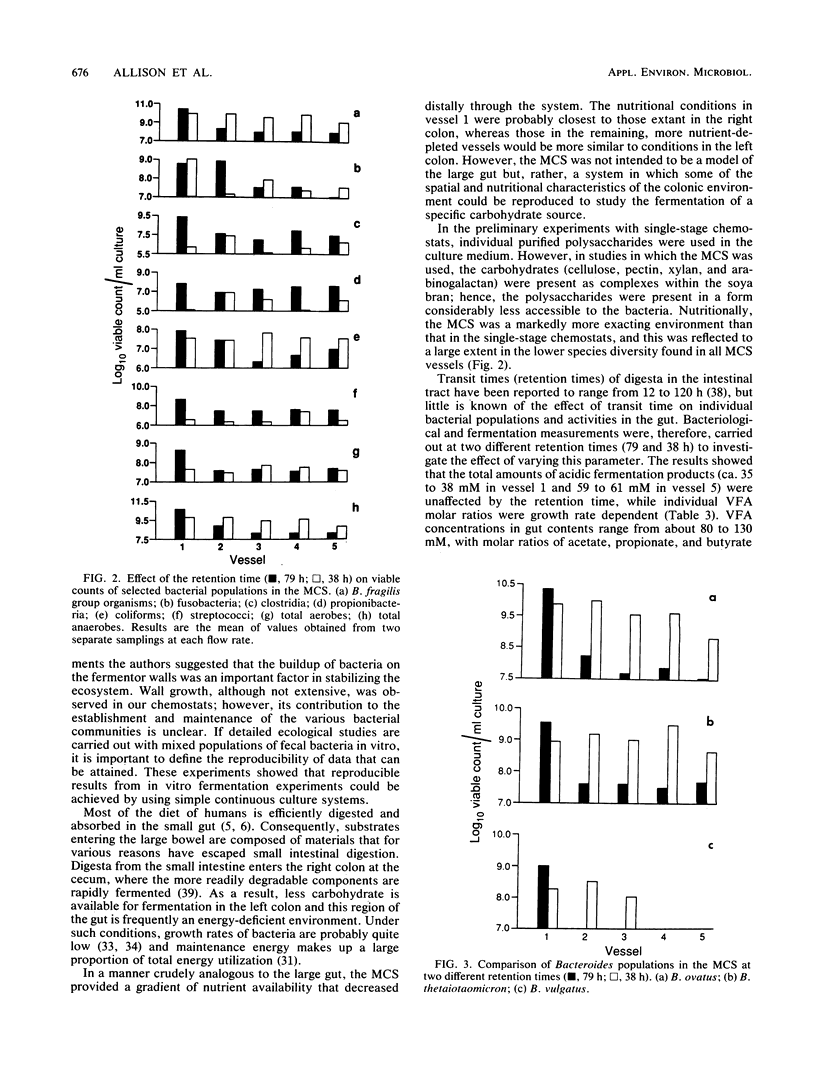
Mixed intestinal bacteria were grown for 336 h in two identical single-stage chemostats at low growth rates in a carbohydrate-limited medium. Complex bacterial populations were maintained and anaerobes always outnumbered aerobes. The predominant organisms belonged to the genera Bacteroides, Bifidobacterium, Lactobacillus, Clostridium, Eubacterium, Propionbacterium, Peptococcus, and Peptostreptococcus. Bacteroides species predominated in both fermentors, particularly B. ovatus and B. thetaiotaomicron. A high degree of reproducibility of bacteriological and fermentation product data was obtained in these experiments. When gut contents were inoculated into a five-stage continuous culture system (retention time of 79 or 38 h) containing soya bran, the medium flow rate had little quantitative effect on the formation of acidic fermentation products; however, more oxidized fermentation acids were produced at the higher retention time. Diverse bacterial populations were maintained in every vessel at each flow rate. Bacteroides fragilis group organisms, especially B. ovatus, were numerically the most important. The viability of bacteria decreased through the system, especially at a retention time of 79 h, when the bacteria were growing under severely nutrient-limited conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Short chain fatty acids in the human colon. Gut. 1981 Sep;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Duerden B. I., Read N. W. Metabolism of mixed human colonic bacteria in a continuous culture mimicking the human cecal contents. Gastroenterology. 1985 Jun;88(6):1903–1909. doi: 10.1016/0016-5085(85)90017-4. [DOI] [PubMed] [Google Scholar]
- Englyst H. N., Cummings J. H. Digestion of polysaccharides of potato in the small intestine of man. Am J Clin Nutr. 1987 Feb;45(2):423–431. doi: 10.1093/ajcn/45.2.423. [DOI] [PubMed] [Google Scholar]
- Englyst H. N., Cummings J. H. Digestion of the carbohydrates of banana (Musa paradisiaca sapientum) in the human small intestine. Am J Clin Nutr. 1986 Jul;44(1):42–50. doi: 10.1093/ajcn/44.1.42. [DOI] [PubMed] [Google Scholar]
- Englyst H., Wiggins H. S., Cummings J. H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982 Mar;107(1272):307–318. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- Fredrickson A. G. Behavior of mixed cultures of microorganisms. Annu Rev Microbiol. 1977;31:63–87. doi: 10.1146/annurev.mi.31.100177.000431. [DOI] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Effect of long generation times on growth of Bacteroides thetaiotaomicron in carbohydrate-induced continuous culture. J Bacteriol. 1981 Jun;146(3):853–860. doi: 10.1128/jb.146.3.853-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein H. C., Snyder M. L. The Inhibition of the Spreading Growth of Proteus and other Bacteria to Permit the Isolation of Associated Streptococci. J Bacteriol. 1941 Nov;42(5):653–664. doi: 10.1128/jb.42.5.653-664.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J. M., Probst I. The biology of gastrointestinal bacteroides. Annu Rev Microbiol. 1979;33:561–594. doi: 10.1146/annurev.mi.33.100179.003021. [DOI] [PubMed] [Google Scholar]
- Malik A. C., Reinbold G. W., Vedamuthu E. R. An evaluation of the taxonomy of Propionibacterium. Can J Microbiol. 1968 Nov;14(11):1185–1191. doi: 10.1139/m68-199. [DOI] [PubMed] [Google Scholar]
- McGeachie J., Kennedy A. C. Simplified quantitative methods for bacteriuria and pyuria. J Clin Pathol. 1963 Jan;16(1):32–38. doi: 10.1136/jcp.16.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Fermentation by the human large intestine microbial community in an in vitro semicontinuous culture system. Appl Environ Microbiol. 1981 Sep;42(3):400–407. doi: 10.1128/aem.42.3.400-407.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Identification of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1306–1313. doi: 10.1093/ajcn/25.12.1306. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A., WISEMAN R. F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951 Jul;62(1):132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick I. G., Levin M. A. Quantitative procedure for enumeration of bifidobacteria. Appl Environ Microbiol. 1981 Sep;42(3):427–432. doi: 10.1128/aem.42.3.427-432.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol. 1979 Mar;37(3):537–543. doi: 10.1128/aem.37.3.537-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Arthur R., Kuritza A. Digestion of larch arabinogalactan by a strain of human colonic Bacteroides growing in continuous culture. J Agric Food Chem. 1981 May-Jun;29(3):475–480. doi: 10.1021/jf00105a009. [DOI] [PubMed] [Google Scholar]
- Salyers A. A. Bacteroides of the human lower intestinal tract. Annu Rev Microbiol. 1984;38:293–313. doi: 10.1146/annurev.mi.38.100184.001453. [DOI] [PubMed] [Google Scholar]
- Scardovi V., Trovatelli L. D. New species of bifid bacteria from Apis mellifica L. and Apis indica F. A contribution to the taxonomy and biochemistry of the genus Bifidobacterium. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1969;123(1):64–88. [PubMed] [Google Scholar]
- Veilleux B. G., Rowland I. Simulation of the rat intestinal ecosystem using a two-stage continuous culture system. J Gen Microbiol. 1981 Mar;123(1):103–115. doi: 10.1099/00221287-123-1-103. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wiggins H. S., Cummings J. H. Evidence for the mixing of residue in the human gut. Gut. 1976 Dec;17(12):1007–1011. doi: 10.1136/gut.17.12.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren M. W. The culture of clinical specimens for anaerobic bacteria: a comparison of three regimens. J Med Microbiol. 1977 May;10(2):195–201. doi: 10.1099/00222615-10-2-195. [DOI] [PubMed] [Google Scholar]


