Abstract
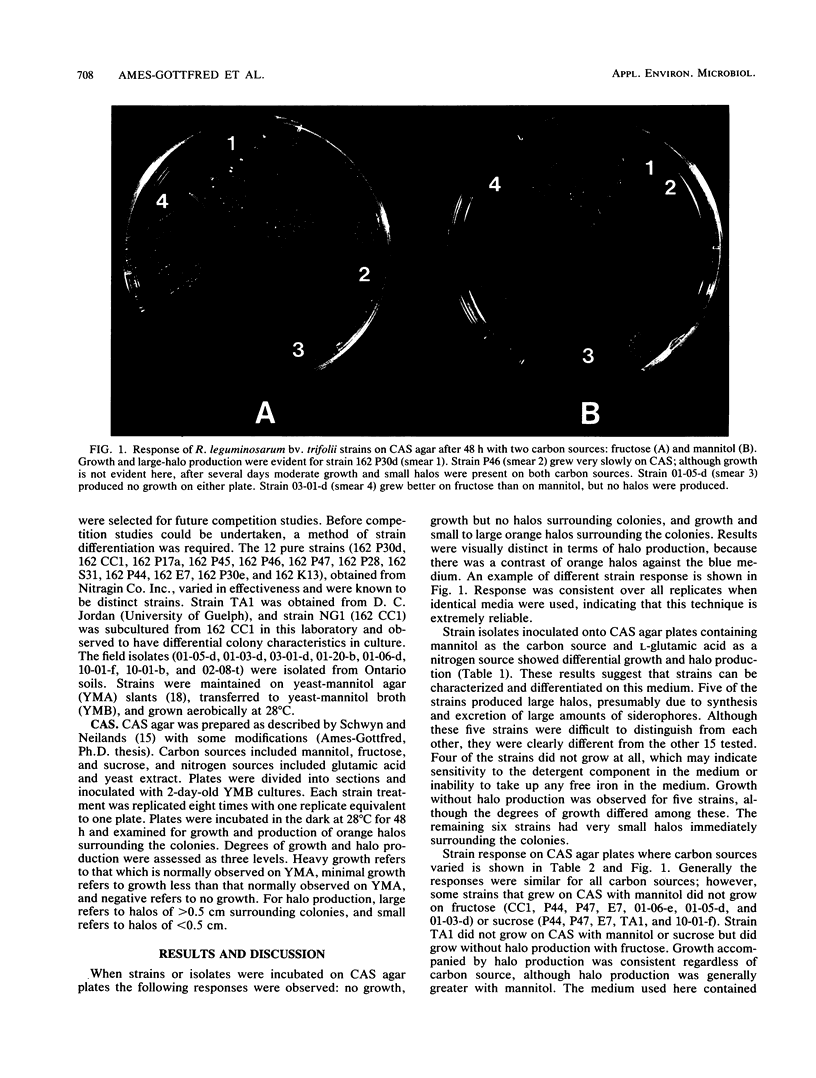
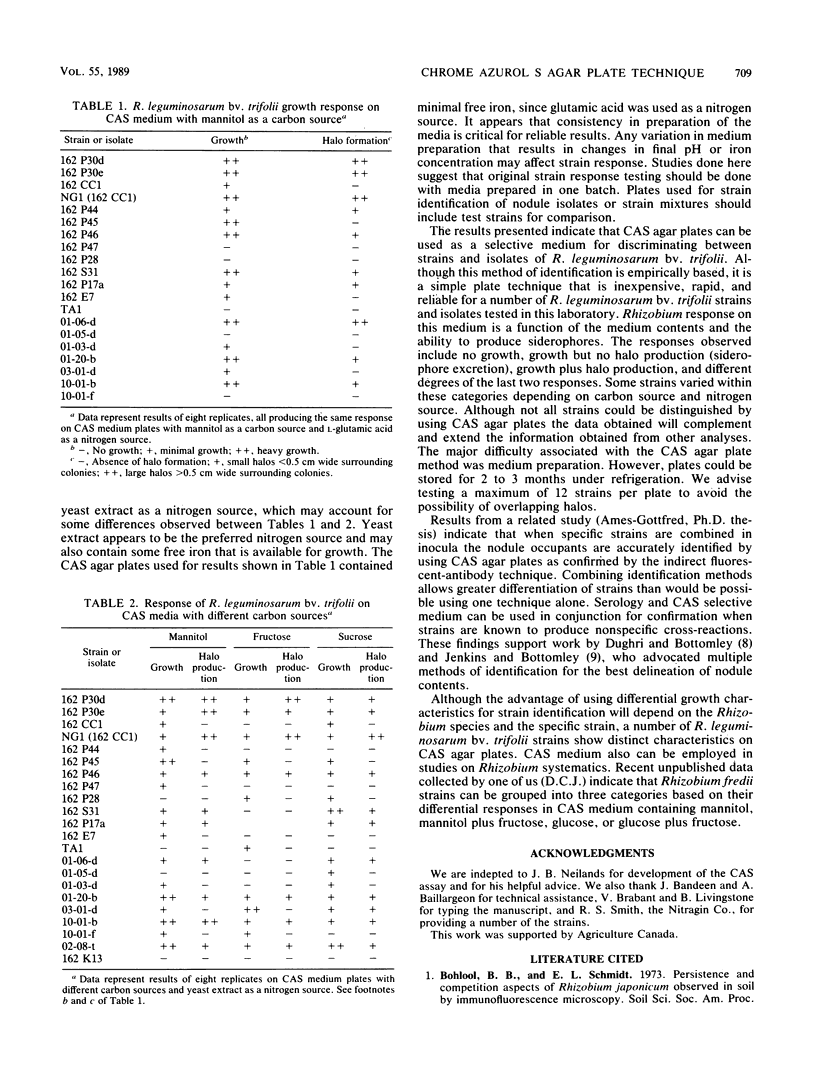
Identification of Rhizobium and Bradyrhizobium strains and especially of indigenous isolates continues to be one of the major difficulties associated with competition studies. Because there is no universally accepted method, the method of choice depends on preference, experience, and equipment. Here, an agar plate technique was used to distinguish strains and field isolates of Rhizobium leguminosarum biovar trifolii to provide a basis for identifying nodule occupants in further competition studies. A rapid plate technique, based on differential growth characteristics, complements other techniques such as serological reactions, particularly when antisera cross-react with nonhomologous strains. The technique involves culturing strains and isolates on chrome azurol S agar. Although similar responses were observed among some strains, the response was highly reproducible and was considered an ideal complementary technique used in conjunction with serological procedures. Strains with similar responses could often be differentiated by varying media components, such as the source of carbon.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUDMAN W. F. IMMUNE DIFFUSION ANALYSIS OF THE EXTRACELLULAR SOLUBLE ANTIGENS OF TWO STRAINS OF RHIZOBIUM MELILOTI. J Bacteriol. 1964 Sep;88:782–794. doi: 10.1128/jb.88.3.782-794.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishinevsky B., Bar-Joseph M. Rhizobium strain identification in Arachis hypogaea nodules by enzyme-linked immunosorbent assay (ELISA). Can J Microbiol. 1978 Dec;24(12):1537–1543. doi: 10.1139/m78-245. [DOI] [PubMed] [Google Scholar]
- MEANS U. M., JOHNSON H. W., DATE R. A. QUICK SEROLOGICAL METHOD OF CLASSIFYING STRAINS OF RHIZOBIUM JAPONICUM IN NODULES. J Bacteriol. 1964 Mar;87:547–553. doi: 10.1128/jb.87.3.547-553.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst C. E. Symbiotic effectiveness of antibiotic-resistant mutants of fast- and slow-growing strains of Rhizobium nodulating Lotus species. Can J Microbiol. 1977 Aug;23(8):1026–1033. doi: 10.1139/m77-152. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]



