Abstract
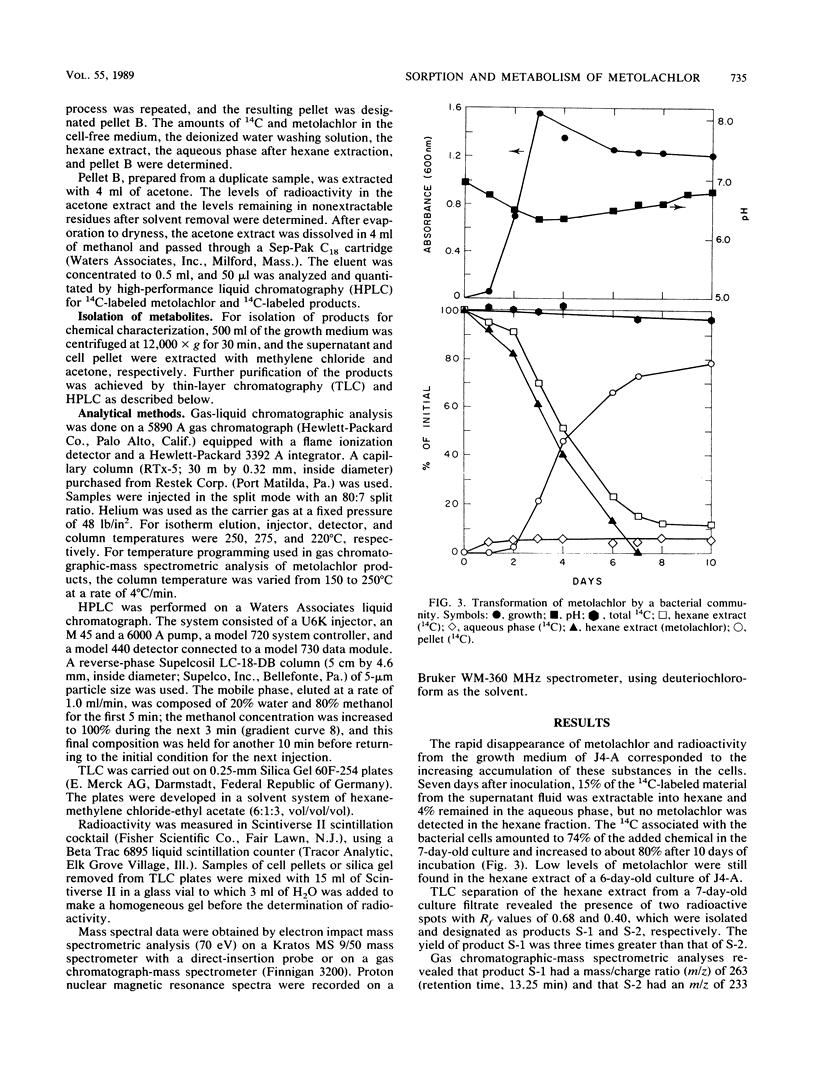
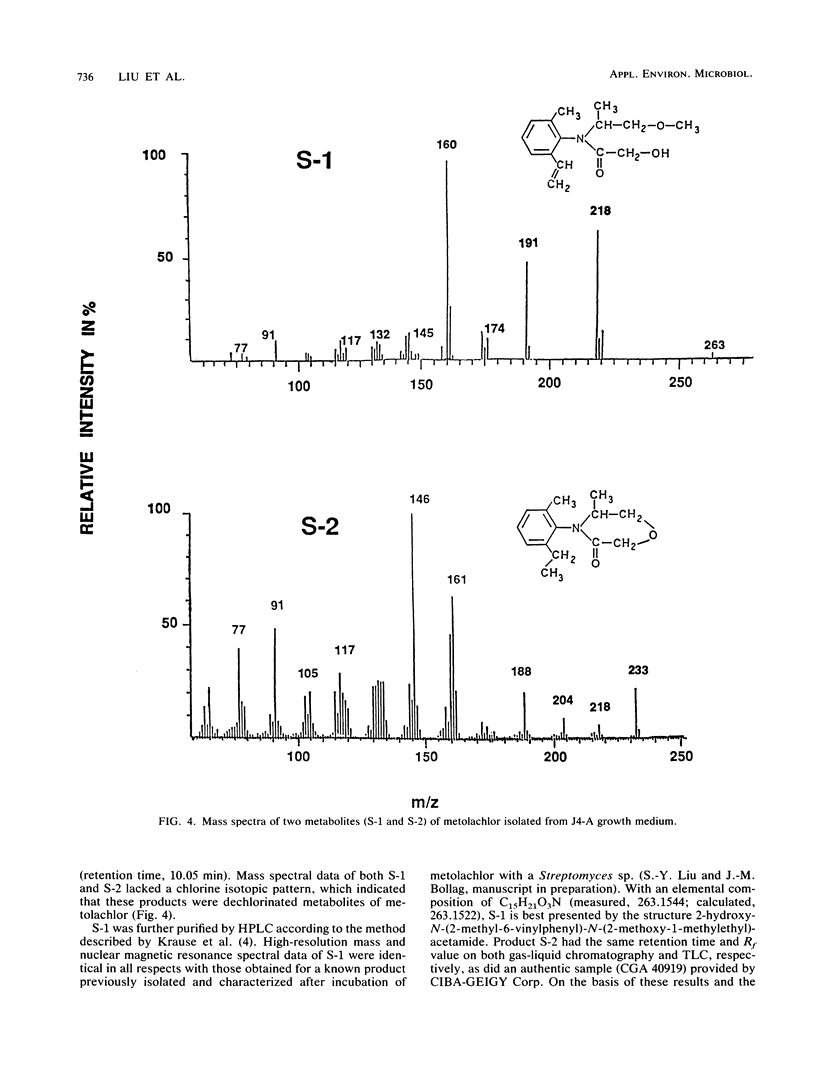
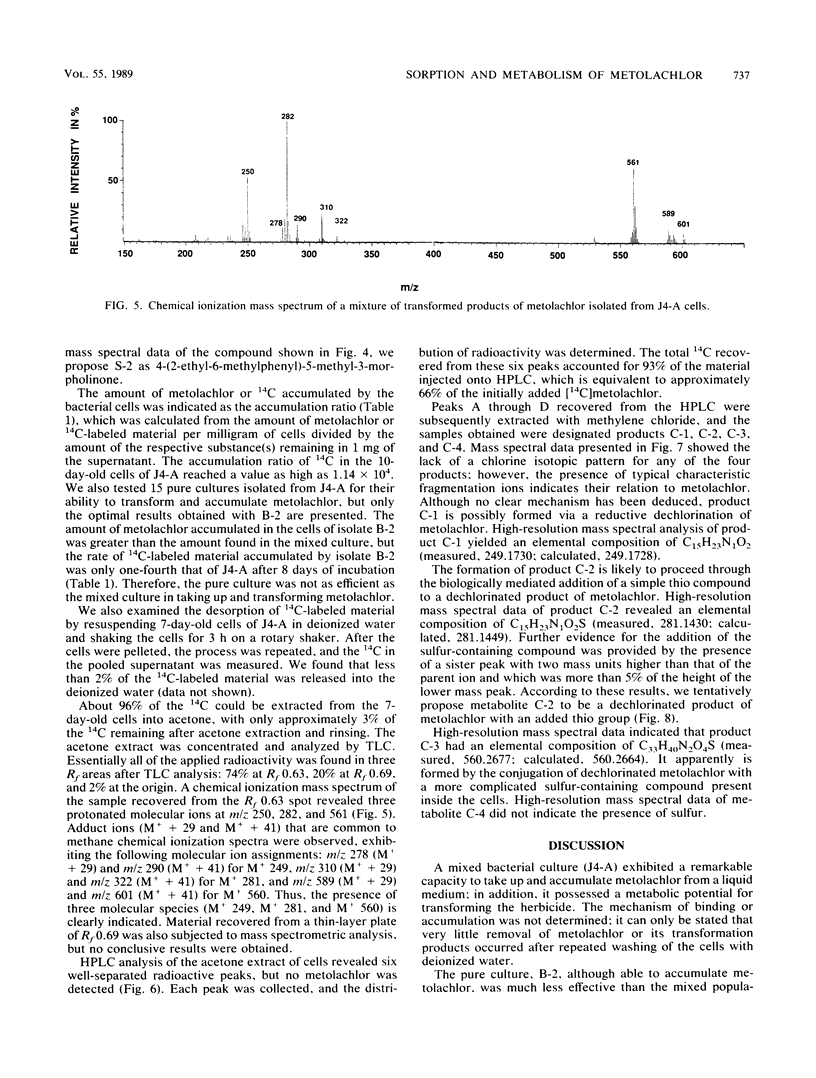
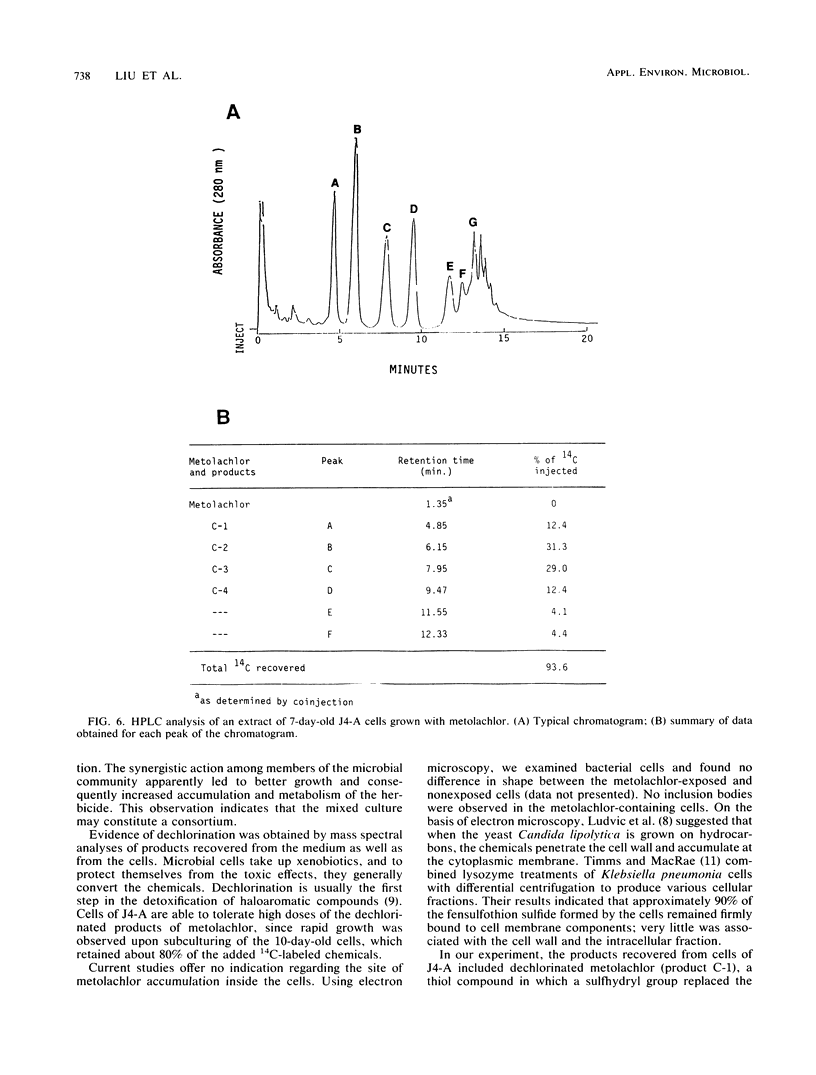
A stable bacterial community absorbed and transformed the herbicide metolachlor [2-chloro-N-(2-ethyl- 6-methylphenyl)-N-(2-methoxy-1-methylethyl)-acetamide] from a liquid medium. About 80% of the added ring-[U-14C]metolachlor (50 μg/ml) disappeared from the medium and accumulated inside the cells. The ratio of cellular 14C to 14C in 1 mg of supernatant reached a value of 1.1 × 104 in a 10-day-old culture. 14C remaining in the medium consisted primarily of two dechlorinated products of metolachlor with m/z 233 and 263 as determined by mass spectrometry. The 14C-labeled material absorbed by the cells was strongly bound; only 2% of the 14C was released into deionized water after shaking for 3 h. Approximately 96% of the 14C associated with the biomass was extracted with acetone, and high-performance liquid chromatographic analysis of this fraction showed six peaks containing radioactivity. Since no metolachlor was detected by chromatographic analysis, it was concluded that the radioactivity recovered from the cells represented transformed products of metolachlor. Pure cultures isolated from the bacterial mixed culture were less effective in transforming and accumulating metolachlor. These results suggest that it may be advantageous to seed an aquatic environment with a mixture of microorganisms, rather than individual microbial species, as a method for removal or detoxification of metolachlor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughman G. L., Paris D. F. Microbial bioconcentration of organic pollutants from aquatic systems -- a critical review. Crit Rev Microbiol. 1981;8(3):205–228. doi: 10.3109/10408418109085079. [DOI] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Kadota H., Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- Lal R., Saxena D. M. Accumulation, metabolism, and effects of organochlorine insecticides on microorganisms. Microbiol Rev. 1982 Mar;46(1):95–127. doi: 10.1128/mr.46.1.95-127.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvík J., Munk V., Dostálek M. Ultrastructural changes in the yeast Candida lipolytica caused by penetration of hydrocarbons into the cell. Experientia. 1968 Oct 15;24(10):1066–1068. doi: 10.1007/BF02138754. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Saxena A., Zhang R. W., Bollag J. M. Microorganisms capable of metabolizing the herbicide metolachlor. Appl Environ Microbiol. 1987 Feb;53(2):390–396. doi: 10.1128/aem.53.2.390-396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms P., MacRae I. C. Conversion of fensulfothion by Klebsiella pneumoniae to fensulfothion sulfide and its accumulation. Aust J Biol Sci. 1982;35(6):661–667. doi: 10.1071/bi9820661. [DOI] [PubMed] [Google Scholar]
- Timms P., MacRae I. C. Reduction of fensulfothion and accumulation of the product, fensulfothion sulfide, by selected microbes. Bull Environ Contam Toxicol. 1983 Jul;31(1):112–115. doi: 10.1007/BF01608775. [DOI] [PubMed] [Google Scholar]


