Abstract
A number of in vitro studies have shown that activation of muscarinic receptors by cholinergic agonists stimulates the nonamyloidogenic, α-secretase-processing pathway of amyloid precursor protein (APP). To determine whether increased cholinergic neurotransmission can modify the APP processing in vivo, we administered a muscarinic receptor agonist (RS86) to normal or aged rats and rats with severe basal forebrain cholinergic deficits (induced by 192 IgG-saporin). The levels of the cell-associated APP in neocortex, hippocampus, and striatum, as well as the secreted form of APP (APPs) in cerebrospinal fluid, were examined by Western blots. Additionally, we investigated the association between the altered APP levels and behavioral deficits caused by cholinergic lesions. We found that treatment with muscarinic receptor agonist resulted in decreased APP levels in neocortex and hippocampus and increased levels of APPs in cerebrospinal fluid. Regulation of APP processing by the muscarinic agonist treatment occurred not only in normal rats, but also in aged and cholinergic denervated rats that model this aspect of Alzheimer’s disease. Interestingly, we found that elevation of APP in neocortex correlated with the cognitive deficits in water-maze testing of rats with cholinergic dysfunction. These data indicate that increased cholinergic neurotransmission can enhance nonamyloidogenic APP processing in intact and lesioned rats and that APP may be involved in cognitive performance.
Progressive loss of cognition and memory is the predominant characteristic of Alzheimer’s disease (AD) (1). In AD, among several pathological features, extracellular deposition of amyloid β peptide (Aβ), neuronal tangles, and marked cholinergic cortical afferent dysfunction are fundamental (2). In spite of an improved understanding of genetic correlates of AD, it is not understood how cognitive deficits, amyloid formation, and cholinergic degeneration may be interrelated. In this regard, a number of questions need to be elucidated, including whether amyloid precursor protein (APP) and its derivatives are involved in cognitive deficits and cholinergic dysfunction and, in turn, how the degenerating cholinergic system may influence the metabolism of APP. Trisomy 21 in Down’s syndrome with an extra copy of the APP gene eventually causes AD-like neuropathology including cholinergic dysfunction and impaired cognition, suggesting a gene dose effect on the cholinergic system and cognitive processes (3, 4). Experimental studies reveal that cholinergic function is altered in trisomy-16 mice, considered an animal model of human Down’s syndrome (5). Genetic studies show that transgenic mice overexpressing human APP can exhibit some learning and memory deficits with or without amyloid plaque formation (6–8), indicating a potential direct role of APP and Aβ in the cognitive deficits. In contrast, secreted forms of APP (APPs) have potent memory-enhancing effects and can block learning deficits induced by scopolamine (9). Intracerebroventricular (ICV) infusion of Aβ induces memory deficits accompanied by cholinergic dysfunction (10, 11), and Aβ can inhibit cholinergic neurotransmitter function without apparent neurotoxicity (12), providing a possible explanation for the involvement of the APP-related system in the cognitive processes. Nevertheless, several studies failed to observe impairments in learning and memory in APP transgenic mice (13, 14), demonstrating that alterations of APP or Aβ may not be sufficient to create major cognitive abnormalities. Nonetheless, these studies suggest that APP and its derivatives participate in cognitive processes and cholinergic hypoactivity. However, the mechanisms involved are not fully understood.
In rats, it has been shown that lesions of the cholinergic nucleus basalis region of the brain with N-methyl-d-aspartate (NMDA) elevate APP mRNA and APP synthesis in the cerebral cortex, and this induction is rapid and persistent (15). Transection of the cholinergic fimbria–fornix pathway also results in marked accumulation of APP in regions of cholinergic fiber denervations in the hippocampus (16). More recently, we and others (17, 18) found that selective depletion of cholinergic neurons in the basal forebrain with 192 IgG-saporin elevated APP immunoreactivity in the cerebral cortex and hippocampus and increased APP levels correlated with decreased cholinergic activity. Furthermore, Rossner et al. (19) observed that levels of APPs decreased and cortical levels of full-length APP concomitantly increased in this cholinergic deafferentation model. Collectively, these observations imply that the integrity of cortical cholinergic function is important for the maintenance of normal APP levels as well as regulation of APP metabolism.
APP can be processed proteolytically via alternative pathways. In the α-secretase pathway, the cleavage occurs within Aβ domain and generates a large, secreted form of APP (APPs) and, therefore, precludes the formation of the amyloidogenic Aβ (20). Cleavages at the N and C termini of Aβ domain by β- and γ-secretase can yield Aβ (21, 22). Proteolytic processing of APP can be regulated by a variety of neurotransmitter receptors, including acetylcholine, serotonin, glutamate, vasopressin, and bradykinin (23). Nitsch et al. (24) found that agonist stimulation of muscarinic receptors by carbachol increased APPs release in transfected cells expressing only M1 and M3 receptor subtypes. Stimulated secretion of APPs by muscarinic receptor activation is accompanied by the reduction of Aβ levels, suggesting a reciprocal relationship between the processing of APP into either a nonamyloidogenic or amyloidogenic pathway (25). The effects of muscarinic M1 and M3 receptors on APP processing are mediated by the phosphoinositide secondary messenger system and protein kinase C (PKC) (26). Activation of PKC with phorbol esters or constitutive up-regulation of PKC in rats results in enhanced secretion of APPs (27, 28), whereas PKC inhibitors block this effect (29, 30). Elevated release of APPs by receptor stimulation is not affected by protein synthesis inhibitors, indicating that preexisting APP is cleaved (31). However, increased APP processing by PKC activation is independent of direct APP phosphorylation, indicating that other proteins are phosphorylated and may be involved in APP processing (32).
These in vitro data are intriguing; however, in vivo experiments have been lacking to determine the effects on APP processing of increased cholinergic neurotransmission. In an attempt to address this issue, we administered a muscarinic receptor agonist (RS86) to normal, aged, and also 192 IgG-saporin-lesioned rats with behavioral deficits. Western blot analysis was used to assay APP levels in different brain regions as well as APPs in cerebrospinal fluid (CSF). In 192 IgG-saporin-lesioned rats, we quantified cognitive deficits in a water-maze task system and their possible relationship to APP levels.
Materials and Methods
Experimental Design.
We conducted three experiments. Experiment 1: a total of 20 adult female Sprague–Dawley rats (250–275 g; Charles River Breeding Laboratories) received 192 IgG-saporin lesions. Fourteen animals received no lesions and served as a control group. Six months postlesion, all animals were tested behaviorally in the Morris water maze. The animals then were divided into four balanced groups based on their latency score before treatment with a muscarinic receptor agonist or vehicle: lesion + agonist (n = 10), lesion + vehicle (n = 10), normal + agonist (n = 7), and normal + vehicle (n = 7). During an 8-day period of pharmacological treatment, the animals once again were tested in the Morris water maze (at 12 months postlesion) and sacrificed on the last day of testing. Experiment 2: a group of normal rats was treated with a muscarinic receptor agonist (n = 10) and vehicle (n = 10). CSF was taken from these rats. Experiment 3: a group of aged (27-month-old) male Fisher 344 rats (450–500 g; National Institute on Aging, Bethesda, MD) was treated with a muscarinic receptor agonist (n = 10) or vehicle (n = 8).
Lesion Surgery.
Adult female Sprague–Dawley rats (n = 20; Charles River Breeding Laboratories) received bilateral ICV injections of 192 IgG-saporin, a mAb directed against the low-affinity nerve growth factor receptor coupled to the ribosome-inactivating protein saporin (Chemicon). Each rat received a total of 5 μg of toxin (1 μg/μl dissolved in sterile PBS), 2.5 μg per site, via a 10-μl Hamilton syringe at the following stereotaxic coordinates: AP, −0.6; L, +/−1.5; V, −3.5; IB, −3.4 (all coordinates are relative to Bregma). All surgeries were performed under aseptic conditions in a Kopf stereotaxic frame (Kopf Instruments, Tujunga, CA). Beginning on the day of lesion surgery and for 1 week afterward, the animals were injected s.c. with sterile saline (0.9%) to combat weight loss and dehydration often associated with the lesion. Unoperated age-matched rats (n = 14) were housed in parallel.
Muscarinic Receptor Agonist Treatment.
RS86 (gift from Novartis, Basel, Switzerland) is a putative muscarinic M1 receptor agonist (33–35). Based on previous studies (33, 34) we selected a range of appropriate doses of RS86 to test in our behavioral paradigm. Rats were injected with different doses of RS86 (1, 2, 3, and 4 mg/kg, respectively, dissolved in sterile saline) and were observed for peripheral side effects and toxicity every 10 min up to 1 hr from the time of injection. The rats injected with the higher doses (3 and 4 mg/kg) showed severe salivation, hypothermia, lacrimation, respiratory distress, and diarrhea within 10 min, peaked at 30 min, and continued for 1 hr after injection. The animals receiving 1 and 2 mg/kg showed minimal side effects and some salivation. Two animals given 3 or 4 mg/kg showed eye irritation at 20 min after injection. The animals received the second injection 4 hr later. All the signs observed in the first injection appeared earlier after the second injection and continued for about 2 hr. Based on these observations, animals in all experiments reported here were injected twice daily with the following doses: 1.5 mg/kg i.m. at approximately 9:00 a.m. and 2.0 mg/kg i.m. 8 hr later, for 8 consecutive days. Control animals received an equal volume of saline (i.m.) at the same time intervals as the RS86-treated groups. All injections were done at the same time every day throughout the experiments.
Behavioral Testing in the Morris Water Maze.
The behavioral testing was carried out in the Morris water maze as described previously by Lin et al. (17) (Poly-Track Video Tracking System; San Diego Instruments, San Diego), starting on the third day of drug treatment. Briefly, a 6-foot tank was filled with water at room temperature and a clear Plexiglas platform was submerged 1–2 inches underwater in the southwest quadrant of the pool. Each rat received six trials per day for 5 consecutive days, and each trial lasted a maximum of 60 sec. Animals were placed randomly in the tank from one of four fixed points (designated North, South, East, and West) and allowed to swim for 60 sec or until they escaped the task by finding the platform. On the last day of testing, the rats received an additional trial (spatial probe trial), where the platform was removed from the tank and the rats were allowed to swim for 60 sec. Total swim time (escape latency), distance to the platform, and time spent in the target quadrant were recorded for each rat for each trial.
Postmortem Procedures.
Immediately before sacrifice, in Experiment 2 the group of normal rats treated with agonist or vehicle was placed in a stereotaxic frame (Kopf Instruments) under anesthesia with sodium pentobarbital (Sigma). The occipital foramen was exposed and a cannula was inserted into the cisterna magna. CSF (≈100 μl) was collected, frozen on dry ice, and kept at −70°C until Western blot assay for secreted form of APP. The brains were then removed and dissected on ice into cerebral cortex, hippocampus, and striatum for Western blot analysis. Animals in Experiment 1 were sacrificed with an overdose of sodium pentobarbital (i.p.). The brains were removed rapidly and the two hemispheres were separated. The left hemisphere was dissected immediately on ice into cerebral cortex, hippocampus, and striatum and stored at −70 for Western blot assay. The right hemispheres were postfixed in 4% phosphate-buffered paraformaldehyde (pH 7.4) at 4°C overnight and then cryoprotected in 20% sucrose in PBS. After equilibration, the brain hemispheres were sectioned coronally on a freezing sliding microtome in six series of 40-μm-thick sections. Animals in Experiment 3 were sacrificed and the brains were dissected as described above for Western blots.
Histology and Immunohistochemistry.
Free-floating sections from Experiment 1 were stained for choline acetyltransferase (ChAT). Using a standard avidin-biotin technique, the sections were incubated overnight at room temperature with primary antibody (1:2,000, polyclonal ChAT; Chemicon), followed by incubation in biotinylated, species-specific secondary antibody (1:200, Vector Laboratories) and avidin-biotin complex (Elite ABC kit; Vector Laboratories), both for 1 hr at room temperature. The sections were then developed in 3,3′-diaminobenzidine tetrahydrochloride dissolved in TBS (Tris-buffered saline; 0.05 M, pH 7.8) with 0.03% hydrogen peroxide added. Adjacent sections were stained for acetylcholinesterase (AChE) activity according to the method of Andra and Lojda (36).
Western Blots.
The antibody 22C11 (Boehringer Mannheim) raised against an N-terminal epitope of APP was used to determine the full-length APP in protein extracts obtained from brain tissues and the secreted form of APP in CSF. In some cases (see Results), Jonas (Boehringer Mannheim), a mAb directed against residues 643–695 in the C terminus of APP, was used to demonstrate the presence of full-length APP in protein extracts obtained from brain tissues as well as the absence of the C terminus in APPs in CSF. The tissue was homogenized by using a hand-held homogenizer in cell lysis buffer (50 mM Tris, pH 8.0/150 mM NaCl/5 mM EDTA/1% Triton X-100/10 μg/ml aprotinin/25 μg/ml leupeptin/10 μg/ml pepstatin/1 mM PMSF; all protease inhibitors were purchased from Sigma) and then sonicated. Homogenates were centrifuged at 14,000 × g for 30 min at 4°C. The supernatant was collected and aliquots were stored at −70°C until assay. The samples of CSF were diluted to a concentration of 1 mg/ml protein with PBS (0.1 M, pH 7.4) and incubated overnight at 4°C with 22C11 (1:1,000). The immunocomplex was captured by incubating with protein G-Sepharose (Sigma) overnight at 4°C. To enhance immunoprecipitation, a bridging rabbit anti-mouse antibody (1:200) was added. The Sepharose beads then were collected by pulse centrifugation for 5 sec at 14,000 × g and washed three times with PBS before being resuspended and dissociated in sample buffer. Samples containing equal amounts of total protein were boiled with SDS sample buffer and electrophoresed on 10% SDS-polyacrylamide gels. Proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Membranes were blocked with 2.5% BSA in 0.1% TBS-T (0.05 M Tris-buffered saline, pH 7.4/0.1% Tween 20) and then were incubated with 22C11 (1:500) overnight at 4°C. The secondary horseradish peroxidase-linked antibody (dilution, 1:6,000; Amersham) was visualized by enhanced chemiluminescence by using Kodak X-Omat films.
Densitometric Analysis.
Quantitation of AChE activity and APP immunoreactive bands was performed by using densitometry. Images of AChE-stained sections were taken in two sections from each brain under the same conditions using a video camera, computer-assisted system (Adobe photoshop program using Macintosh computer, Video Lumina Leaf system, Southborough, MA). Each region was sampled in duplicate within a standard box created by the computer monitor at 10× for cerebral cortex and 5× for dorsal hippocampus. Optical density (OD) was calculated by using an image analysis program (nih image 1.61). Films of Western blots were scanned (Scanner ASTRA 1200S, UMAX Systems, Freemont, CA) by using Adobe photoshop 4 (Adobe Systems, Mountain View, CA), and the OD of the APP bands was measured by using nih image 1.61. The relative APP values were calculated by subtracting the background OD value from the measured OD of the APP bands. Groups used for statistical analyses were always determined within the same Western blot. Typically, the results were confirmed by duplicate.
Statistical Analysis.
All statistical analyses were carried out by using the jmp program (version 3.1.6; SAS Institute, Cary, NC). The behavioral data were assessed by ANOVA and followed by Tukey–Kramer posthoc analysis when significant F-ratios were present. Densitometric data were compared between different groups for each brain region with an unpaired Student’s t test, and differences between groups were considered statistically significant when P < 0.05. Regression analyses were performed with linear fit for two independent variables (jmp 3.1.6; SAS Institute).
Results
Effects of 192 IgG-Saporin Lesions and Muscarinic Receptor Agonist on Behavior.
Consistent with previous findings (37), bilateral ICV injections of 192 IgG-saporin against the low-affinity nerve growth factor receptor caused a severe and permanent reduction of AChE activity in cerebral cortex and hippocampus and a dramatic loss of ChAT neurons in the basal forebrain (90–95%). To determine the effects of muscarinic receptor agonist on memory performance, all animals in Experiment 1 treated with the muscarinic receptor agonist or saline were tested in the Morris water maze at 12 months postlesion. In the time to escape to the platform (escape latency), the rats treated with muscarinic receptor agonist did not significantly differ from those of rats treated with saline in either normal or 192-saporin-lesioned group during testing [for the statistical interaction: treatment × days, F(4, 9) = 0.18 and F(4, 15) = 0.21, P > 0.05, respectively]. In the spatial probe trial at the end of training trials, all lesioned rats spent significantly less time in the target quadrant than normal rats (Table 1) and muscarinic receptor agonist treatments did not have any positive effect. Swim speed was not affected by the treatment throughout the testing [F(4, 29) = 0.2, P > 0.05]. Notably, the percent time spent in the target quadrant correlated with postmortem AChE activity in the dorsal hippocampus in the lesioned rats treated with saline (regression analysis: r = 0.66, n = 10; P < 0.05).
Table 1.
Behavioral performance in the Morris water maze 12 months after 192 IgG-saporin lesions and RS86 treatment
| Group | Escape latency on day 5, sec | % time spent in target quadrant on spatial probe trial |
|---|---|---|
| Normal + saline | 20 ± 4 | 55 ± 5 |
| Normal + RS86 | 26 ± 8 | 55 ± 7 |
| Lesion + saline | 30 ± 4 | 37 ± 5* |
| Lesion + RS86 | 36 ± 5 | 39 ± 2* |
Data are presented as group mean ± SEM. One-way ANOVA (F3.33 = 4.16, P < 0.05) followed by post-hoc Student’s t test; *, P < 0.05, significantly different from normal saline-treated rats by Student’s t test.
192 IgG-Saporin Lesions and APP Western Blot Analysis.
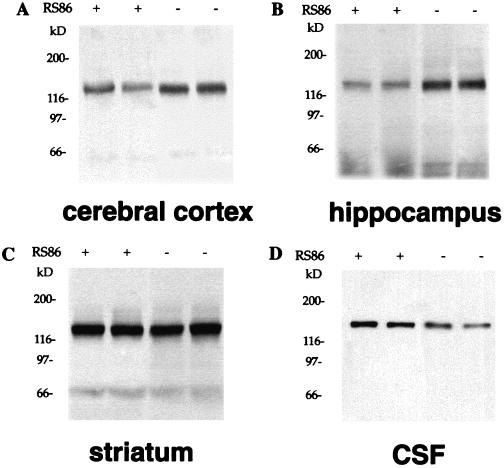
In the present study, the molecular mass of APP detected with 22C11 in protein extracts obtained from brain tissue is approximately 120 kDa (Fig. 1). This APP protein is similar to those identified as cell-associated, full-length of APP in rats (38). An antibody (Jonas) directed against the C terminus of APP (39) reacted strongly with protein extracts prepared with the same procedure, demonstrating the presence of full-length APP in these samples.
Figure 1.
Representative Western blots of APP with 22C11 in normal rats. (A) Levels of cell-associated, full-length APP were reduced in the presence of RS86 (indicated by +) related to those in the absence of RS86 (indicated by −) in cerebral cortex. (B) Decreased levels of cell-associated, full-length APP are found in the presence of RS86 (+) related to those in the absence (−) of RS86 in hippocampus. (C) No such changes were observed in the presence (+) and absence (−) of RS86 in striatum. (D) Secreted form of APP in CSF was immunoprecipitated and probed with 22C11. The levels of APPs were increased by RS86 (+) compared with vehicle (−).
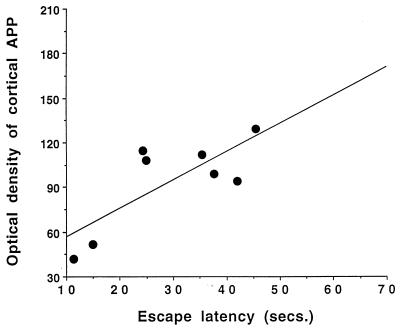
In several cases 12 months post-192 IgG-saporin lesion, Western blot analysis revealed that APP levels were increased in the cerebral cortex and hippocampus (Table 2), but these changes did not reach statistical significance in this study when compared with unoperated, aged-matched controls. Also, APP levels in the striatum did not show significant changes in lesioned rats compared with controls (Table 2). Interestingly, regression analysis showed that APP levels in the cerebral cortex positively correlated with the escape latency score obtained on day 5 in the water maze testing (regression analysis: r = 0.77, n = 8; P < 0.05) in lesioned rats treated with saline (Fig. 2). No such correlation was found in unoperated control rats.
Table 2.
APP levels in rats 12 months after 192 IgG-saporin lesion
| Region | Cerebral cortex | Hippocampus | Striatum |
|---|---|---|---|
| APP, % of normal control | 114 ± 13 | 124 ± 21 | 56 ± 17 |
Data are presented as mean ± SEM APP OD values in different regions of the brain and are expressed as percentage of APP values in normal control group.
Figure 2.
Correlation of cortical APP levels (OD values) with escape latency scores obtained on day 5 in a water maze testing in 192 IgG-saporin-lesioned, saline-treated rats (regression analysis: r = 0.77, n = 8; P < 0.05).
Effects of Muscarinic Receptor Agonist on APP Metabolism.
To determine the effects of muscarinic receptor agonist on APP metabolism, the groups of normal, 192 IgG-saporin-lesioned, and aged rats were treated with a muscarinic receptor agonist (RS86) identically (3.5 mg/kg per day, i.m., for 8 days). The postmortem cell-associated, full-length APP were measured in tissue homogenates obtained from cerebral cortex, hippocampus, and striatum. The APPs was detected in CSF of normal rats.
In normal rats, Western blots analysis revealed that APP levels were reduced significantly by 28% (P = 0.0002) in cerebral cortex and 45% (P = 0.0001) in hippocampus of rats treated with muscarinic receptor agonist compared with those treated with vehicle (Table 3). No such changes were found in striatum (Table 3). To measure the levels of APPs in CSF, samples of CSF were immunoprecipitated and immunoblotted with 22C11. The levels of APPs were increased significantly in CSF treated with muscarinic receptor agonist compared with vehicle controls (Table 4). To confirm that the APP detected with 22C11 in CSF is secreted in amino-terminal derivatives of APP, Jonas was used to examine APP in CSF. No obvious immunoreactive bands were found in CSF with this antibody (data not shown), demonstrating that the secreted form of APP in CSF detected in the present study lacks C terminus as described previously (40).
Table 3.
Effect of RS86 on APP levels in normal rats
| Region | Cerebral cortex | Hippocampus | Striatum |
|---|---|---|---|
| APP, % of normal control | 72 ± 5* | 55 ± 5* | 123 ± 9 |
Data are presented as mean ± SEM APP OD values in different regions of the brain and are expressed as percentage of APP values in normal, vehicle-treated (saline) control group. *, P < 0.05, significantly different from the vehicle-treated control group (Student’s t test).
Table 4.
Effect of RS86 on APPs in the CSF of normal rats
| Sample | Saline | RS86 |
|---|---|---|
| CSF | 106 ± 18 | 156 ± 12* |
CSF samples were immunoprecipitated and then immunoblotted with 22C11. Data represent mean ± SEM APPs OD values. *, P < 0.05, significantly different from the vehicle-treated control group (Student’s t test).
Effects of muscarinic receptor agonist on APP levels in 192 IgG-saporin-lesioned rats are shown in Table 5. In these rats, treatment with muscarinic receptor agonist induced a dramatic reduction of APP in the hippocampus (47%; P = 0.0001). However, the average reduction of APP levels seen in cerebral cortex (12%) and striatum (29%) was not significant.
Table 5.
Effect of RS86 on APP levels in rats 12 months after 192 IgG-saporin lesions
| Region | Cerebral cortex | Hippocampus | Striatum |
|---|---|---|---|
| APP, % of lesion control | 88 ± 13 | 53 ± 6* | 71 ± 15 |
Data are presented as mean ± SEM APP OD values in different regions of the brain and are expressed as percentage of APP values in lesioned, vehicle-treated control group. *, P < 0.05, significantly different from vehicle-treated control group (Student’s t test).
Effect of Muscarinic Receptor Agonist on APP in Aged Rats.
To investigate whether the effects of muscarinic receptor agonist on APP levels found in normal rats described above could occur in aged rats; an experiment was conducted in a set of 27-month-old male fisher 334 rats. As in the previous experiments, reduction of APP protein occurred in cerebral cortex and hippocampus (44% and 37%; P < 0.05, respectively) in rats treated with muscarinic receptor agonist compared with age-matched vehicle controls (Table 6). APP changes in the striatum were not measured in these rats.
Table 6.
Effect of RS86 on APP levels in aged rats (27 months)
| Region | Cerebral cortex | Hippocampus | Striatum |
|---|---|---|---|
| APP, % of aged control | 56 ± 9* | 63 ± 10* | N/A |
Data are presented as mean ± SEM APP OD values in different regions of the brain and are expressed as percentage of APP values in aged, vehicle-treated (saline) control group. N/A, not analysis. *, P < 0.05, significantly different from aged, vehicle-treated control group (Student’s t test).
Discussion
The present study shows that in normal, aged, and 192 IgG-saporin-lesioned rats, treatment with a muscarinic receptor agonist results in decreases in levels of the cell-associated, full-length APP in cerebral cortex and/or hippocampus and concomitant increases in levels of APPs in CSF. These results indicate that APP processing can be regulated by in vivo muscarinic receptor stimulation. Furthermore, we find that APP levels correlate with behavioral deficits in 192 IgG-saporin-lesioned rats, suggesting that APP or APP-related systems may also be involved in the cognitive performance (9, 23). However, there were no positive effects of muscarinic receptor agonist treatment on behavioral performance with our experimental parameters.
192 IgG-Saporin Lesions and APP.
Selective degeneration of the basal forebrain cholinergic neurons and amyloid deposition are central features in AD. A determination of how these two events may be interrelated in the development of AD becomes critical to understanding the pathogenesis of AD. In rats, injection of excitotoxins into nucleus basalis magnocellularis results in increased synthesis of both APP mRNA and APP protein in cholinergic-denervated cerebral cortex (15, 41), and transection of fimbria–fornix similarly leads to increased immunoreactivity of APP in the hippocampus (16). Moreover, APP accumulation is observed only in aged rats displaying behavioral deficits associated with reduced cholinergic activity (16). These studies indicate that loss of cholinergic innervation can alter APP expression. More recently, a novel immunotoxin, 192 IgG-saporin, was developed to selectively destroy cholinergic neurons in the basal forebrain. This also provides a powerful tool to elucidate the relationship between cholinergic dysfunction and the APP-related system. We previously observed elevations in APP immunoreactivity in neocortex and hippocampus in rats with 192 IgG-saporin lesions (17). This finding was replicated by another investigator (18), who also confirmed that APP changes are negatively correlated with AChE activity. In many cases of saporin-lesioned rats in the present study, APP levels measured by Western blot analysis were elevated, but the average levels were not increased in a statistical analysis. Increased APP immunoreactivity in neocortex and hippocampus was found in a regional and cell layer-specific pattern (17, 18). These regional elevations therefore may not be able to significantly change the average APP levels of the entire neocortex and hippocampus after selective cholinergic deafferentation.
Effect of Muscarinic Receptor Agonist on APP Levels and APP Processing.
The molecular and cellular mechanisms involved in the induction of APP by lesions of the cholinergic system are not well understood. Some studies suggest that reactive glial cells play a role in the induction of APP (16, 42, 43). Levels of total APP mRNA are not altered significantly in rats with 192 IgG-saporin lesions (19, 44), indicating that the increases in APP levels may be due to abnormal APP processing. Rossner et al. (19) found that reduced cholinergic activity by partial cholinergic lesions led to increased levels of the cortical full-length APP, and restoration of cholinergic activity by ICV transplantation of NGF-secreting fibroblast normalized the cortical APP levels, demonstrating that APP processing may be under cholinergic control. Until recently, however, few studies explored the effects of muscarinic receptor activation on APP metabolism in vivo. Here we show that, in rats, administration of a muscarinic receptor agonist results in significant decreases in APP levels in cerebral cortex and hippocampus as well as increases in APPs levels in CSF, indicating that treatment with muscarinic receptor agonist leads to an enhanced α-secretase pathway of APP processing. Notably, the regulation of APP processing by this treatment is found not only in normal rats but also in aged and cholinergic lesioned rats with behavioral deficits, which are analogous to those seen in AD. These findings suggest that proteolytic processing of APP can be regulated by treatment with a muscarinic receptor agonist under these in vivo conditions.
APP processing is believed to play a central role in the amyloidogenesis of AD and Down’s syndrome. Multiple external and internal signals can regulate APP processing (45). A number of reports demonstrate that muscarinic acetylcholine receptors play a significant role in APP processing. Nitsch et al. (24) found that agonist stimulation of muscarinic receptors by carbachol increases APPs release in transfected cells expressing M1 or M3, but not M2 or M4 receptor subtype, indicating that the regulation of APP processing is receptor subtype-specific (46). Further studies show that activation of muscarinic receptor not only accelerates APPs secretion, but also inhibits Aβ formation (25, 47). M1 and M3 receptor subtypes are coupled to G protein and mediate their effects through the phosphoinositide cascade in which PKC is stimulated, whereas M2 and M4 subtypes are associated with the inhibition of adenylate cyclase (26). Activation of PKC by phorbol esters greatly enhances the release of APPs, whereas inhibition of PKC can block this effect (25, 30, 48, 49). Because PKC is involved in protein phosphorylation, it raises a question of whether activation of PKC increases phosphorylation of APP that leads to enhanced cleavage of APP. Recent evidence shows that activation of PKC does not increase APP phosphorylation, suggesting that APP regulation is independent of direct phosphorylation and, in fact, APP secretion proceeds in the absence of either cytoplasmic or extracellular phosphorylation (48). These observations suggest that PKC activation led to phosphorylation and activation of additional kinases, which are involved in the regulation of APP processing (32). Regardless of the mechanisms involved in the regulation of APP processing, our findings extend the in vivo observations of cholinergic regulation of APP metabolism and provide evidence that restoration of cholinergic function by increased cholinergic transmission can enhance the nonamyloidogenic pathway of APP processing. However, the absence of positive effects of this treatment on behavioral performance may be due to the peripheral side effects induced by this drug.
Correlation Between Cholinergic System, APP, and Behavioral Deficits.
Interestingly, we found that increased APP levels in cerebral cortex correlated with cognitive deficits observed in a water maze in 192 IgG-saporin-lesioned rats. Originally, this could be a direct reflection of the loss of cholinergic input and its effect on cognition (17, 18). However, it has also been shown that transgenic mice overexpressing several isoforms of human APP develop age-dependent learning and memory deficits (7, 8, 50, 51). Transgenic mice expressing the 751-aa isoform of human APP display aged-dependent deficits in spatial learning in a water maze task and in spontaneous alteration in a Y maze along with Aβ deposition and aberrant tau protein expression (7). Moreover, Hsiao et al. (8) found some memory deficits in parallel with Aβ elevation and amyloid plaques in transgenic mice overexpressing the mutant 695-aa isoform of human APP, which resemble those seen in AD (8). Remarkably, cognitive deficits are observed along with Aβ accumulation as well as cell loss in the hippocampus in mice expressing the normal C-terminal 104 aa of APP (51). Also, memory impairment is found in APP transgenic mice without significant elevation of Aβ (6). These genetic studies suggest a potential role of elevated APP or Aβ in cognitive deficits. In contrast, APPs, thought to have neurotrophic and neuroprotective effects, can improve memory performance and block the memory deficits caused by scopolamine (9). Taken together with the data shown here, this implies that APP and its derivatives may participate in cognitive processes. However, the mechanism whereby Aβ is linked to the cognitive impairment characteristic of AD remains unclear. In addition to neurotoxic effects, Aβ can potently inhibit cholinergic neurotransmission at low concentrations (picomolar and nanomolar) without apparent neurotoxicity, indicating that this capacity of Aβ might contribute to the dysfunction of cholinergic system associated with the memory impairment in AD (52–54). Additionally, reduced glucose utilization in hippocampus was observed in APP transgenic mice with behavioral deficits (50). A similar observation has been made in AD patients showing reduced levels of central nervous system-specific glucose transporters (55). We also found significant decreases in glucose utilization in a number of regions including cortex and hippocampus in 192 IgG-saporin-lesioned rats with behavioral deficits (unpublished data), suggesting a role of impaired cellular energy systems in altered APP processing and cognitive deficits in AD.
In this study we determined that cognitive deficits induced by cholinergic lesions correlate with APP protein levels in the cerebral cortex and, moreover, that cholinergic muscarinic receptors participate directly in the regulation of APP metabolism. These findings provide a new perspective to the relationships between dementia, Aβ deposition, and cholinergic dysfunction in the progression of AD. The observed reductions of APP protein levels and increase in APPs secretion after muscarinic agonist treatment suggest that the use of selective M1 receptor agonists or other pharmacological agents to restore normal cholinergic function could be of value toward accelerating nonamyloidogenic processing, thereby reducing Aβ load in patients with AD and Down’s syndrome. Alternatively, the biological restoration of cholinergic hypoactivity by intracerebral cholinergic cell transplantation potentially would ameliorate behavioral deficits as well as reduce Aβ formation. These hypotheses merit further investigation.
Acknowledgments
We thank Novartis for providing the compound of RS86, Dr. Julian Gray for helpful discussions, and Ms. Sandra Pohlman for excellent administration. This work was supported by the Irving and Betty Brudnick Research Fund at McLean Hospital.
Abbreviations
- APP
amyloid precursor protein
- APPs
secreted form of amyloid precursor protein
- ICV
intracerebroventricular
- PKC
protein kinase C
- AD
Alzheimer’s disease
- Aβ
amyloid β peptide
- CSF
cerebrospinal fluid
- AChE
acetylcholinesterase
References
- 1.Terry R D, Katzman R. Ann Neurol. 1983;14:497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- 2.Coyle J T, Price D L, DeLong M R. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 3.Epstein C J. The Neurobiology of Down Syndrome. New York: Raven; 1986. [Google Scholar]
- 4.Epstein C J. The Consequences of Chromosomal Imbalance, Principles, Mechanisms, Models. New York: Cambridge Univ. Press; 1986. [Google Scholar]
- 5.Fiedler J L, Epstein C J, Rapoport S I, Caviedes R, Caviedes P. Brain Res. 1994;658:27–32. doi: 10.1016/s0006-8993(09)90006-3. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb L, Gordon M N, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, et al. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 7.Moran P M, Higgins L S, Cordell B, Moser P C. Proc Natl Acad Sci USA. 1995;92:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;272:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 9.Meziane H, Dodart J-C, Mathis C, Little S, Clemens J, Paul S M, Ungerer A. Proc Natl Acad Sci USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabeshima T, Nitta A. Tohoku J Exp Med. 1994;174:241–249. doi: 10.1620/tjem.174.241. [DOI] [PubMed] [Google Scholar]
- 11.Maurice T, Lockhart B P, Privat A. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 12.Auld D S, Kar S, Quirion R. Trends Neurosci. 1998;21:43–49. doi: 10.1016/s0166-2236(97)01144-2. [DOI] [PubMed] [Google Scholar]
- 13.Borchelt D R, Ratovitski T, Lare J V, Lee M K, Gonzales V, Jinkins N A, Copeland N G, Price D L, Sisodia S S. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 14.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T. Nature (London) 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 15.Wallace W, Ahlers S T, Gotlib J, Bragin V, Sugar J, Gluck R, Shea P A, Davis K L, Haroutunian V. Proc Natl Acad Sci USA. 1993;90:8712–8716. doi: 10.1073/pnas.90.18.8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeson J G, Shelton E R, Chan H W, Gage F H. J Comp Neurol. 1994;342:69–77. doi: 10.1002/cne.903420108. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Leblanc C, Deacon T, Isacson O. NeuroReport. 1998;9:547–552. doi: 10.1097/00001756-199802160-00030. [DOI] [PubMed] [Google Scholar]
- 18.Leanza G. Neurosci Lett. 1998;257:53–56. doi: 10.1016/s0304-3940(98)00744-7. [DOI] [PubMed] [Google Scholar]
- 19.Rossner S, Ueberham U, Yu J, Kirazov L, Reinhard S, Perez-Polo J R, Bigl V. Eur J Neurosci. 1997;9:2125–2134. doi: 10.1111/j.1460-9568.1997.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 20.Esch F S, Keim P S, Beattie E C, Blacher R W, Culwell A R, Oltersdorf T, McClure D, Ward P J. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 21.Haass C, Schlossmacher M G, Hung A Y, Vigo-Pelfrey C, Mellon A, Ostaszewski B L, Lieberburg I, Koo E H, Schenk D, Teplow D B, et al. Nature (London) 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 22.Shoji M, Golde T E, Ghiso J, Cheung T T, Estus S, Shaffer L M, Cai X-D, McKay D M, Tintner R, Frangione B, Younkin S G. Science. 1992;258:126–327. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 23.Roberson M R, Harrell L. Brain Res Rev. 1997;25:50–69. doi: 10.1016/s0165-0173(97)00016-7. [DOI] [PubMed] [Google Scholar]
- 24.Nitsch R M, Slack B E, Wurtman R J, Growdon J H. Science. 1992;258:304–310. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 25.Hung A Y, Haass C, Nitsch R M, Qiu W Q, Citron M, Wurtman R J, Growdon J, Selkoe D J. J Biol Chem. 1993;268:22959–22962. [PubMed] [Google Scholar]
- 26.Nitsch R M, Growdon J H. Biochem Pharmacol. 1994;47:1275–1284. doi: 10.1016/0006-2952(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 27.Caputi A, Barindelli S, Pastorino L, Cimino M, Buxbaum J D, Cattabeni F, Luca M D. J Neurochem. 1997;68:2523–2529. doi: 10.1046/j.1471-4159.1997.68062523.x. [DOI] [PubMed] [Google Scholar]
- 28.Slack B E, Nitsch R M, Livneh E, Kunz G M, Jr, Breu J, Eldar H, Wurtman R J. J Biol Chem. 1993;268:21097–21101. [PubMed] [Google Scholar]
- 29.Nitsch R M, Deng M, Growdon J H, Wurtman R J. J Biol Chem. 1996;271:4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- 30.Slack B E, Breu J, Petryniak M A, Srivastava K, Wurtman R J. J Biol Chem. 1995;270:8337–8344. doi: 10.1074/jbc.270.14.8337. [DOI] [PubMed] [Google Scholar]
- 31.Nitsch R M, Kim C, Growdon J H. Neurochem Res. 1998;23:807–814. doi: 10.1023/a:1022423813362. [DOI] [PubMed] [Google Scholar]
- 32.Hung A Y, Selkoe D J. EMBO J. 1994;13:534–542. doi: 10.1002/j.1460-2075.1994.tb06291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupniak N M J, Tye S J, Iversen S D. J Neurol Sci. 1992;110:222–227. doi: 10.1016/0022-510x(92)90031-f. [DOI] [PubMed] [Google Scholar]
- 34.Wanibuchi F, Konishi T, Harada M, Terai M, Hidaka K, Tamura T, Tsukamoto S-i, Usuda S. Eur J Pharmacol. 1990;187:479–486. doi: 10.1016/0014-2999(90)90374-f. [DOI] [PubMed] [Google Scholar]
- 35.Palacios J M, Bolliger G, Closse A, Enz A, Gmelin G, Malanowski J. Eur J Pharmacol. 1986;125:45–62. doi: 10.1016/0014-2999(86)90082-8. [DOI] [PubMed] [Google Scholar]
- 36.Andra J, Lojda Z. Histochemistry. 1986;84:575–579. doi: 10.1007/BF00482994. [DOI] [PubMed] [Google Scholar]
- 37.Leanza G, Nilsson O G, Wiley R G, Bjorklund A. Eur J Neurosci. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 38.Neve R L, Rogers J, Higgins G A. Neuron. 1990;5:329–338. doi: 10.1016/0896-6273(90)90169-g. [DOI] [PubMed] [Google Scholar]
- 39.Jung S S, Nalbantoglu J, Cashman N R. J Neurosci Res. 1996;46:336–348. doi: 10.1002/(SICI)1097-4547(19961101)46:3<336::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Palmert M R, Podlisny M B, Witker D S, Oltersdorf T, Younkin L H, Selhoe D J, Younkin S G. Proc Natl Acad Sci USA. 1989;86:6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace W, Bragin V, Robakis N K, Sambamurti K, VanderPutten D, Merril C R, Davis K L. Mol Brain Res. 1991;10:173–178. doi: 10.1016/0169-328x(91)90108-a. [DOI] [PubMed] [Google Scholar]
- 42.Kalaria R N, Bhatti S U, Palatinsky E A, Pennington D H, Shelton E R, Chan H W, Perry G, Lust W D. NeuroReport. 1993;4:211–214. doi: 10.1097/00001756-199302000-00025. [DOI] [PubMed] [Google Scholar]
- 43.Banati R B, Gehrmann J, Kreutzberg G W. NeuroReport. 1994;5:1359–1361. doi: 10.1097/00001756-199406270-00016. [DOI] [PubMed] [Google Scholar]
- 44.Apelt J, Schliebs R, Beck M, Robner S, Bigl V. Int J Dev Neurosci. 1996;15:95–112. doi: 10.1016/s0736-5748(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 45.Nitsch R M. Neurodegeneration. 1996;5:477–482. doi: 10.1006/neur.1996.0066. [DOI] [PubMed] [Google Scholar]
- 46.Nitsch R M, Blusztaji J K, Pittas A G, Slack B E, Growdon J H, Wurtman R J. Proc Natl Acad Sci USA. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobsen J S, Spruyt M A, Brown A M, Sahasrabudhe S R, Blume A J, Vitek M P, Muenkel H A, Sonnenberg-Reines J. J Biol Chem. 1994;269:8376–8382. [PubMed] [Google Scholar]
- 48.Gabuzda D, Busciglio J, Yankner B A. J Neurochem. 1993;61:2326–2329. doi: 10.1111/j.1471-4159.1993.tb07479.x. [DOI] [PubMed] [Google Scholar]
- 49.Caporaso G L, Gandy S E, Buxbaum J D, Ramabhadran T V, Greengard P. Proc Natl Acad Sci USA. 1992;89:3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsiao K K, Borchelt D R, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younki S, Price D, et al. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 51.Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner S A, Massicotte G, Julien J-P, et al. Nature (London) 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 52.Kar S, Seto D, Gaudreau P, Quirion R. J Neurosci. 1996;16:1034–1040. doi: 10.1523/JNEUROSCI.16-03-01034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kar S, Issa A M, Seto D, Auld D S, Collier B, Quirion R. J Neurochem. 1998;70:2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- 54.Kelly J F, Furukawa K, Barger S W, Rengen M R, Mark R J, Blanc E M, Roth G S, Mattson M P. Proc Natl Acad Sci USA. 1996;93:6753–6758. doi: 10.1073/pnas.93.13.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson I A, Chundu K R, Davies-Hill T, Honer W G, Davies P. Ann Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]